��Ŀ����
���������ѧ֪ʶ�������ͼ��ʾװ�ûش��й����⣺
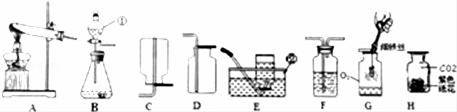

��1��ָ��������������ƣ�������������
��2���ø��������ȡ�����Ļ�ѧ����ʽΪ������Ӧѡ��ķ���װ��������������˫��ˮ��ȡ��������Ӧѡ��ķ���װ�����������䷴Ӧԭ�����������û�ѧ��Ӧ����ʽ��ʾ����
��3��������Eװ���ռ�������ԭ�������������������ļ���ƿӦ��������������������������ϣ�
��4����ȡ���ռ�һ�����Ķ�����̼��Ӧѡ��ķ������ռ�װ���������䷴Ӧԭ�����������û�ѧ��Ӧ����ʽ��ʾ����
��5�����ռ����������Ͷ�����̼�ֱ������ͼG��H��ʾʵ�飬G�м���ƿ�ײ�ʢ������ˮ��ԭ����������H��������ɫʯ����Һ��ֽ��������ɫ��
�����㡿��������ķ���װ�ú��ռ�װ����ѡȡ�����������Ļ�ѧ���ʣ�ʵ������ȡ�����ķ�Ӧԭ����������̼��ʵ�����Ʒ���������̼�Ļ�ѧ���ʣ���д��ѧ����ʽ�����ֱ���ʽ�����뷽��ʽ��
��ר�⡿���������ʵ�����Ʒ������顢�����뾻����
����������1��Ҫ��Ϥ�������������ơ���;��ʹ�÷�����
��2�������������ʱ�ܹ��ֽ���������ء��������̺����������������ڶ������̵Ĵ������·ֽ�Ϊˮ��������
��3��ʵ����ͨ���ô���ʯ��ʯ��ʯ��ϡ���ᷴӦ��ȡ������̼����Ӧ����Ҫ���ȣ�����ʯ��ʯ��ʯ����Ҫ�ɷ���̼��ƣ��ܺ�ϡ���ᷴӦ�����Ȼ��ơ�ˮ�Ͷ�����̼��������̼�ܹ�����ˮ���ܶȱȿ�����Ũ���������ˮ�ԣ���������������������������̼������ĸ������
��4������ʵ��������ȡ������̼�ķ�Ӧԭ���ͷ�Ӧ������ѡ��Ӧ�ķ���װ�ã����з������
��5������������ȼ��ʱ�γɵĸ��������������䣻������̼�ܺ�ˮ��Ӧ����̼�ᣬ̼����ʹʯ����Һ���ɫ��
����𡿽⣺��1�����dz���©����ͨ������©��������Ӧ������ע��Һ��ҩƷ������ˮ�ۣ���������ˮ���ռ����壻
��2���ø��������ȡ�����Ļ�ѧ����ʽΪ��2KMnO4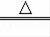
 K2MnO4+MnO2+O2������Ϊ��Ӧ��Ҫ���ȣ�Ӧ����Aװ����Ϊ����װ�ã����������ڶ������̵Ĵ������·ֽ�Ϊˮ����������ӦΪ��Һ���·�Ӧ������װ��ѡ��B����Ӧ�ķ���ʽΪ��2H2O2
K2MnO4+MnO2+O2������Ϊ��Ӧ��Ҫ���ȣ�Ӧ����Aװ����Ϊ����װ�ã����������ڶ������̵Ĵ������·ֽ�Ϊˮ����������ӦΪ��Һ���·�Ӧ������װ��ѡ��B����Ӧ�ķ���ʽΪ��2H2O2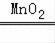
 2H2O+O2��
2H2O+O2��
��3��������Eװ���ռ�������ԭ����������������ˮ�����������ļ���ƿӦ�����������ϣ����������ܶȱȿ�����
��3��ʵ������ȡ������̼����Ҫ���ȣ�Ӧ����Bװ����Ϊ����װ�ã�������̼�ܹ�����ˮ����������ˮ���ռ����ܶȱȿ��������������ſ������ռ�������Dװ���ռ������Ƶø���Ķ�����̼�����轫����ͨ��ʢ��Ũ�����ϴ��ƿF��
��4��ʵ��������ȡ������̼���ô���ʯ��ʯ��ʯ��ϡ���ᷴӦ����ȡ�����ڹ�Һ�����ͣ�����Ӧ��ѡ��Bװ������ȡ������̼���壻������̼���ܶȱȿ�����������ˮ��ֻ���������ſ������ռ�����Ӧ�Ļ�ѧ����ʽ�ǣ�CaCO3+2HCl=CaCl2+H2O+CO2����
��5����˿��O2��ȼ��ʱ��Ϊ��ֹ�������ۻ���������ʹƿ��ը�ѣ�����ƿ�ĵײ�Ӧ��������ˮ����һ��ϸɳ��������̼��ˮ��Ӧ����̼�ᣬ̼����ʹ��ɫʯ����Һ���ɫ��Gƿ����ɫʯ����Һ��ֽ���Ժ�ɫ��
��4��G�м���ƿ�ײ�ʢ����ˮ��ԭ���Ƿ�ֹ�����ۻ��ヲ��ը�Ѽ���ƿ��H��������ɫʯ����Һ��ֽ���Ժ�ɫ��
�����ֹ�����ۻ��ヲ��ը�Ѽ���ƿ����
�ʴ�Ϊ����1������©����ˮ�ۣ�
��2��2KMnO4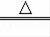
 K2MnO4+MnO2+O2����A��B��2H2O2
K2MnO4+MnO2+O2����A��B��2H2O2
 2H2O+O2��
2H2O+O2��
��3��������������ˮ������
��4��BD��CaCO3+2HCl=CaCl2+H2O+CO2����
��3����ֹ�������ۻ���������ʹƿ��ը�ѣ��죮
���������������ʵ�飬��ѧ�ؽ���ʵ�顢����ʵ�飬�ǵó���ȷʵ����۵�ǰ�ᣬ���Ҫѧ�����ʵ�顢����ʵ�顢����ʵ�飬Ϊѧ�û�ѧ֪ʶ�춨������
��

 ��У����ϵ�д�
��У����ϵ�д�
 B��
B��
 C��
C��
 D��
D��