��Ŀ����
(7��) ij��һ�����ʵ����ǵ���ιۣ��ó���Ҫ��Ʒ֮һ��С�մ�̼�����ƣ����ι۽�����ͬѧ�Ǵ���һЩ�������������С�մ���Ʒ�����ⶨ����̼�����Ƶ������������������Ʒ��ֻ�����Ȼ���һ�����ʣ���ȡ��Ʒ9.3 g��μ���ϡ���ᣬ����CO2�����������μ�ϡ�����������ϵ����ͼ��ʾ�����������ðٷ�����ʾ��������С�����һλ���֣�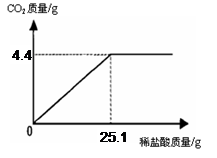
��1����Ʒ��̼�����Ƶ�����������
��2��ǡ����ȫ��Ӧʱ��������Һ�����ʵ�����������
34��(7��)
�⣺����Ʒ��̼�����Ƶ�����Ϊx, �����Ȼ��Ƶ�����Ϊy
 NaHCO3 + HCl NaCl + CO2��+ H2O��1�֣�
NaHCO3 + HCl NaCl + CO2��+ H2O��1�֣�
84 58.5 44
x y 4.4 g
x �� 8.4 g��1�֣�
y �� 5.85 g��1�֣�
(1) ��Ʒ��̼�����Ƶ���������Ϊ 90.3%��2�֣�
(2) ǡ����ȫ��Ӧʱ��������Һ�����ʵ���������Ϊ��22.5%��2�֣�����:
��
�⣺����Ʒ��̼�����Ƶ�����Ϊx, �����Ȼ��Ƶ�����Ϊy
 NaHCO3 + HCl NaCl + CO2��+ H2O��1�֣�
NaHCO3 + HCl NaCl + CO2��+ H2O��1�֣�84 58.5 44
x y 4.4 g
x �� 8.4 g��1�֣�
y �� 5.85 g��1�֣�
(1) ��Ʒ��̼�����Ƶ���������Ϊ 90.3%��2�֣�
(2) ǡ����ȫ��Ӧʱ��������Һ�����ʵ���������Ϊ��22.5%��2�֣�����:
��

��ϰ��ϵ�д�
 99��1������ĩ��ѵ��ϵ�д�
99��1������ĩ��ѵ��ϵ�д� ��ǿ��У��ĩ���100��ϵ�д�
��ǿ��У��ĩ���100��ϵ�д� �óɼ�1��1��ĩ���100��ϵ�д�
�óɼ�1��1��ĩ���100��ϵ�д� ��״Ԫ���źþ�ϵ�д�
��״Ԫ���źþ�ϵ�д�
�����Ŀ
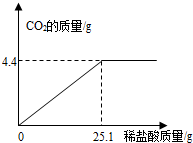 ij��һ�����ʵ����ǵ����Ƹۼ�ιۣ��ó���Ҫ��Ʒ֮һ��С�մ�̼�����ƣ����ι۽�����ͬѧ�Ǵ���һЩ�������������С�մ���Ʒ�����ⶨ����̼�����Ƶ������������������Ʒ��ֻ�����Ȼ���һ�����ʣ���ȡ��Ʒ9.3g��μ���ϡ���ᣬ����CO2�����������μ�ϡ�����������ϵ��ͼ��ʾ�����������ðٷ�����ʾ��������С�����һλ���֣�
ij��һ�����ʵ����ǵ����Ƹۼ�ιۣ��ó���Ҫ��Ʒ֮һ��С�մ�̼�����ƣ����ι۽�����ͬѧ�Ǵ���һЩ�������������С�մ���Ʒ�����ⶨ����̼�����Ƶ������������������Ʒ��ֻ�����Ȼ���һ�����ʣ���ȡ��Ʒ9.3g��μ���ϡ���ᣬ����CO2�����������μ�ϡ�����������ϵ��ͼ��ʾ�����������ðٷ�����ʾ��������С�����һλ���֣�