��Ŀ����
 ��1�����к�̼Ԫ�ص������У������л������
��1�����к�̼Ԫ�ص������У������л������A��̼��� B���Ҵ���C2H5OH �� C��������̼
��2����ʯȼ����Ҫ����ú��
Ҫ�ɷ���
��3����ѧԴ������������̺������ѧ֪ʶ���밴��Ҫ�������գ�����һ�̶��벻���������������������彡��ֱ����أ������пɹ����������������
��4��ʳ����Լ����3%-5%�Ĵ��ᣨC2H4O2����������
��5����ͼ��ʾ������ƿ�����˱�ǩ���Լ�������һƿ��ϡ���ᡢһƿ��ϡ���ᣮΪ��������ƿ��Һ��Ӧѡ�õ��Լ���
���㣺�л��������������,�����ijɷּ����ɷֵ��������,��������Ⱦ����Σ��,��Ļ�ѧ����,��ѧʽ����д������,��д��ѧ����ʽ�����ֱ���ʽ�����뷽��ʽ,��ʯȼ�ϼ����ۺ�����
ר�⣺��ѧ����Դ,��ѧ����������غ㶨��,���ʵķ���,������ˮ,�������� ���ͨ��
��������1���������ʵ���ɷ������ʵ����
��2�����ݻ�ʯȼ�ϵ����༰��Ȼ������Ҫ�ɷֻش�
��3�����ݿ����ijɷֺ���ȾԴ�����ش�
��4�����ݴ���Ļ�ѧʽCH3COOH�����ø�Ԫ�ص����ԭ��������������ԭ�Ӹ��������м��㣮
��5���������ᡢ����ļ��������ش�
��2�����ݻ�ʯȼ�ϵ����༰��Ȼ������Ҫ�ɷֻش�
��3�����ݿ����ijɷֺ���ȾԴ�����ش�
��4�����ݴ���Ļ�ѧʽCH3COOH�����ø�Ԫ�ص����ԭ��������������ԭ�Ӹ��������м��㣮
��5���������ᡢ����ļ��������ش�
����⣺��1���Ҵ���C2H5OH ���Ǻ���̼Ԫ�صĻ���������л����̼��ơ�������̼����Ȼ����̼Ԫ�أ�������ͭ�������ƣ����������
��2����ʯȼ����Ҫ����ú��ʯ�ͺ���Ȼ�������Ƕ�����̼Ԫ�أ�������Ȼ������Ҫ�ɷ���CH4��
��3���������ܹ���������������������Ŀǰ�Ϻ����������ձ���Ҫ��Ⱦ�����������һ����̼������������
��4�����ݴ���Ļ�ѧʽC2H4O2����������C��H��O����Ԫ����ɣ������Ħ������=12��2+1��4+16��2=60g/mol��0.5mol C2H4O2������Լ������ԭ�Ӹ���=6.02��1023��0.5��2���T6.02��1023����
��5��Ϊ������ƿ�����˱�ǩ��ϡ�����ϡ���ᣬȡ��Ʒ�μ��Ȼ�����Һ��������ְ�ɫ����ԭ��ҺΪϡ���ᣬ�����������ԭ��ҺΪϡ���ᣬ�����ķ�Ӧ�ķ���ʽ�ǣ�BaCl2+H2SO4�TBaSO4��+2HCl��
�ʴ�Ϊ����1��B��
��2��ʯ�ͣ�CH4��
��3��������һ����̼��
��4������60g/mol��6.02��1023��0.5��2���T6.02��1023����
��5���Ȼ�����Һ��BaCl2+H2SO4�TBaSO4��+2HCl��
��2����ʯȼ����Ҫ����ú��ʯ�ͺ���Ȼ�������Ƕ�����̼Ԫ�أ�������Ȼ������Ҫ�ɷ���CH4��
��3���������ܹ���������������������Ŀǰ�Ϻ����������ձ���Ҫ��Ⱦ�����������һ����̼������������
��4�����ݴ���Ļ�ѧʽC2H4O2����������C��H��O����Ԫ����ɣ������Ħ������=12��2+1��4+16��2=60g/mol��0.5mol C2H4O2������Լ������ԭ�Ӹ���=6.02��1023��0.5��2���T6.02��1023����
��5��Ϊ������ƿ�����˱�ǩ��ϡ�����ϡ���ᣬȡ��Ʒ�μ��Ȼ�����Һ��������ְ�ɫ����ԭ��ҺΪϡ���ᣬ�����������ԭ��ҺΪϡ���ᣬ�����ķ�Ӧ�ķ���ʽ�ǣ�BaCl2+H2SO4�TBaSO4��+2HCl��
�ʴ�Ϊ����1��B��
��2��ʯ�ͣ�CH4��
��3��������һ����̼��
��4������60g/mol��6.02��1023��0.5��2���T6.02��1023����
��5���Ȼ�����Һ��BaCl2+H2SO4�TBaSO4��+2HCl��
�����������漰��֪ʶ��϶࣬������Ķ��ǿα��Ļ���֪ʶ��ֻҪ��ǿ����֪ʶ��ѧϰ�����ɽ�����⣮

��ϰ��ϵ�д�
 ���Ӣ��������ϵ�д�
���Ӣ��������ϵ�д�
�����Ŀ
�������������л�����ǣ�������
| A��������̼ | B��̼��� |
| C���ƾ� | D��̼�� |
���������ڡ���������������á������������ɫ�����ɻ�ѧ�仯���ֳ������ǣ�������
| A����Ļʽ����� |
| B����ɫ��ͼ�� |
| C����ɫ���й��� |
| D��ҹ������� |
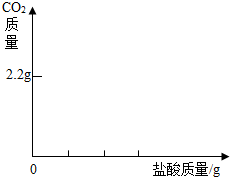 ijѧ���ú���̼�������ʵ��ռ���Ʒ�����ᷢ����Ӧ��ȡ��13.3g�������ƹ�����Ʒ��������ˮ�����Һ�������м���200g 7.3%��ϡ���ᣬʹ���ַ�Ӧ�����ɶ�����̼2.2g����������ȫ���ݳ�����
ijѧ���ú���̼�������ʵ��ռ���Ʒ�����ᷢ����Ӧ��ȡ��13.3g�������ƹ�����Ʒ��������ˮ�����Һ�������м���200g 7.3%��ϡ���ᣬʹ���ַ�Ӧ�����ɶ�����̼2.2g����������ȫ���ݳ�����
 ��������ͼ��ʾװ�ã�β�����������ԣ�����ȡһ����̼�������Բⶨijͭ����Ʒ������CuO��ĩ���н���ͭ�ĺ�����
��������ͼ��ʾװ�ã�β�����������ԣ�����ȡһ����̼�������Բⶨijͭ����Ʒ������CuO��ĩ���н���ͭ�ĺ����� ��ͼ��ʾ�Ǽס����������ʵ��ܽ�����ߣ���ش��������⣺
��ͼ��ʾ�Ǽס����������ʵ��ܽ�����ߣ���ش��������⣺