��Ŀ����
15����������������������ʱ�ڣ�ÿ����Ҫ��ȡ�����ĵ����ʣ������ʵĴ�л������Ҫ������[CO��NH2��2]������ʳ������ȡ�ĵ����ʾ������³´�л����ȫת��Ϊ�����ų����⣬ÿ��ÿ���൱���ų�����30g����1������[CO��NH2��2]��̼���⡢��������Ԫ�������ȣ�3��1��7��4
��2��30g�����к���Ԫ�ض��ٿˣ�
��3�����ٿ��������60g����������Ԫ�������൱��
���� ��1�����ݻ������и�Ԫ��������=��ԭ�ӵ����ԭ��������ԭ�Ӹ���֮�ȣ����з������
��2�����ݻ������ijԪ�ص�����=�û�������������Ԫ�ص��������������з������
��3�����ݻ����������=�û�������ijԪ�ص������¸�Ԫ�ص��������������з������
��� �⣺��1��������̼���⡢������Ԫ�ص�������Ϊ12����1��2��2������14��2����16=3��1��7��4��
��2��30g�����к���Ԫ�ص�����Ϊ30g��$\frac{14��2}{12+16+��14+1��2����2}$��100%=14g��
��3����Ҫ����淋�����Ϊx��x��$\frac{14��2}{��14+1��4����2+32+16��4}��$100%=60g��$\frac{14��2}{12+16+��14+1��2����2}$��100% x=132g��
�ʴ�Ϊ����1��3��1��7��4����2��30g�����к���Ԫ��14g����3��132g�������60g����������Ԫ�������൱��
���� �����ѶȲ�����ͬѧ�ǽ������Ϣ��������û�ѧʽ���йؼ�����з������⡢��������������

��ϰ��ϵ�д�
 ��1����Ԫ�¿�������ĩϵ�д�
��1����Ԫ�¿�������ĩϵ�д�
�����Ŀ
10��������ˮ���������У�����Ҫ��ˮ�м����������������ǣ�������
| A�� | �Ѵ������ʵ�ˮ��ɴ����� | B�� | ��ˮ�������� | ||
| C�� | ʹ��������� | D�� | ���Ӷ������������Ԫ�� |
4�� ��ǰ���в�����Сѧ���С�����ʳ�á����̣����ΪijУʳ��ij����Ͳ���ʳ�ף�
��ǰ���в�����Сѧ���С�����ʳ�á����̣����ΪijУʳ��ij����Ͳ���ʳ�ף�
��1����ʳ���и��������ʵ��Ǻ���ţ�⣬������Ҫ��������������Ӫ���������࣮
���߲˿�Ϊ�����ṩ����ά���أ�ʳ�òɹ����߲�����һ��������������ģ����������߲˵�Ӫ��Һ�䷽��Ҫ�ɷ��У�Ca��NO��3��KNO3��KH2PO4����NH4��2SO4���䷽�����ڸ��Ϸ��ϵ���KNO3��KH2PO4���ѧʽ����
��ʳ�����治��������ù��Ĵ���C��
A����ˮϴ�����������ʳ�á����� B��������������ʳ�á��� C�����Բ���ʳ��
��2��ʳ�ó����������ˣ�ͼ1��
��������������Ҫ���������ĵ����ԣ�
��ʹ����������Ϊ���岹����Ԫ�أ�����ʱ����������ʳ��Ч�����ã���ԭ��2CH3COOH+Fe=��CH3COO��2Fe+H2�����÷���ʽ��ʾ����
������˵��һ�������з�ֹ��������ķ������ֽྻ�����������ɣ���
��3��ʳ��ʹ�õ�ʳ�γ�Ϊ�ӵ��Σ�ʳ���мӵ���Ϊ��Ԥ����״���״���ͼ2��һ���ӵ�ʳ�μ����ǩ������ϸ�Ķ���ǩ���ش��������⣺
�١�ÿ100g����2��6mg���ġ��⡱ָ���ǵ�C����д��ţ���
A������ B������ C��Ԫ�� D��ԭ��
�����ʵ��������������3.4mg����أ��������ȷ��0.1��
��4��ʳ�������ڵ�ζ���Ͼ���ʱ��ζ������ᣬ��������֪���ϾƱ�������Ϊ�Ͼ��еĴ���˾�ʹ�Ҵ�������������Ӧ�����˴����ˮ��
�ٸ÷�Ӧ�Ļ�ѧ��Ӧ����ʽΪC2H5OH+O2$\frac{\underline{\;����˾�\;}}{\;}$CH3COOH+H2O���÷�Ӧ�з�����Ч���õ�����C2H5OH��O2��д�����ķ��ţ���
�ڴ�������ɵĽǶȿ����Ҵ��ʹ���������л���������л������
�۴������������ζƷ�⣬���������кܶ����ã�����ʹ��ʳ�϶����ܴﵽĿ����D��
A����ȥ�˵��ϵ����� B����ȥˮ�����ϵ�ˮ�� C����ϴ����Ƥ���ϵ�ʯ��ˮ D������ƽ���Ʒ���Ƿ���ͭ
��5��Ϊ�˱�֤ͬѧ�ǵ���ʳ������ʳ��ÿ�춼����84����Һ����������84����Һ��һ���д̼�����ζ�ĸ�ЧҺ������������Ҫ�ɷ�Ϊ�������ƣ�NaClO�����������Ƶ���ȡԭ�����������������Ʒ�ӳ�����Ȼ��ơ��������ƺ�ˮ���÷�Ӧ�ķ���ʽΪ��2NaOH+Cl2�TNaCl+H2O+NaClO��
��6��ͬѧ�Ǹ�ѧУʳ�õ����н��鲻��������B
A������ʳ�в������ B�����ṩ��ըʳ�� C���ʵ��ṩˮ��
��7�����л�����ʳƷ��ȫ������2009��6��1����ʵʩ���������������彡��������B
A���þ�����ϩ���ϴ���װʳƷ
B��Ϊ��ֹ�������ɣ�Ӧ��ʳ������Ʒ�����ࡢϺƤ��ʳ��
C����ҵ�����������ƴ���ʳ�Σ�����ζƷ��
D���ø������֣���ȩ��ˮ��Һ�����������ʳƷ��
 ��ǰ���в�����Сѧ���С�����ʳ�á����̣����ΪijУʳ��ij����Ͳ���ʳ�ף�
��ǰ���в�����Сѧ���С�����ʳ�á����̣����ΪijУʳ��ij����Ͳ���ʳ�ף�| ��ʳ | ��� | �ز� |
| �� | ����ţ�� | �����ܲ������ƹ� |
���߲˿�Ϊ�����ṩ����ά���أ�ʳ�òɹ����߲�����һ��������������ģ����������߲˵�Ӫ��Һ�䷽��Ҫ�ɷ��У�Ca��NO��3��KNO3��KH2PO4����NH4��2SO4���䷽�����ڸ��Ϸ��ϵ���KNO3��KH2PO4���ѧʽ����
��ʳ�����治��������ù��Ĵ���C��
A����ˮϴ�����������ʳ�á����� B��������������ʳ�á��� C�����Բ���ʳ��
��2��ʳ�ó����������ˣ�ͼ1��
��������������Ҫ���������ĵ����ԣ�
��ʹ����������Ϊ���岹����Ԫ�أ�����ʱ����������ʳ��Ч�����ã���ԭ��2CH3COOH+Fe=��CH3COO��2Fe+H2�����÷���ʽ��ʾ����
������˵��һ�������з�ֹ��������ķ������ֽྻ�����������ɣ���
��3��ʳ��ʹ�õ�ʳ�γ�Ϊ�ӵ��Σ�ʳ���мӵ���Ϊ��Ԥ����״���״���ͼ2��һ���ӵ�ʳ�μ����ǩ������ϸ�Ķ���ǩ���ش��������⣺
�١�ÿ100g����2��6mg���ġ��⡱ָ���ǵ�C����д��ţ���
A������ B������ C��Ԫ�� D��ԭ��
�����ʵ��������������3.4mg����أ��������ȷ��0.1��
��4��ʳ�������ڵ�ζ���Ͼ���ʱ��ζ������ᣬ��������֪���ϾƱ�������Ϊ�Ͼ��еĴ���˾�ʹ�Ҵ�������������Ӧ�����˴����ˮ��
�ٸ÷�Ӧ�Ļ�ѧ��Ӧ����ʽΪC2H5OH+O2$\frac{\underline{\;����˾�\;}}{\;}$CH3COOH+H2O���÷�Ӧ�з�����Ч���õ�����C2H5OH��O2��д�����ķ��ţ���
�ڴ�������ɵĽǶȿ����Ҵ��ʹ���������л���������л������
�۴������������ζƷ�⣬���������кܶ����ã�����ʹ��ʳ�϶����ܴﵽĿ����D��
A����ȥ�˵��ϵ����� B����ȥˮ�����ϵ�ˮ�� C����ϴ����Ƥ���ϵ�ʯ��ˮ D������ƽ���Ʒ���Ƿ���ͭ
��5��Ϊ�˱�֤ͬѧ�ǵ���ʳ������ʳ��ÿ�춼����84����Һ����������84����Һ��һ���д̼�����ζ�ĸ�ЧҺ������������Ҫ�ɷ�Ϊ�������ƣ�NaClO�����������Ƶ���ȡԭ�����������������Ʒ�ӳ�����Ȼ��ơ��������ƺ�ˮ���÷�Ӧ�ķ���ʽΪ��2NaOH+Cl2�TNaCl+H2O+NaClO��
��6��ͬѧ�Ǹ�ѧУʳ�õ����н��鲻��������B
A������ʳ�в������ B�����ṩ��ըʳ�� C���ʵ��ṩˮ��
��7�����л�����ʳƷ��ȫ������2009��6��1����ʵʩ���������������彡��������B
A���þ�����ϩ���ϴ���װʳƷ
B��Ϊ��ֹ�������ɣ�Ӧ��ʳ������Ʒ�����ࡢϺƤ��ʳ��
C����ҵ�����������ƴ���ʳ�Σ�����ζƷ��
D���ø������֣���ȩ��ˮ��Һ�����������ʳƷ��
 ��ˮ�����ǵ�����ϢϢ��ء�����ش�������ˮ�йص����⣮
��ˮ�����ǵ�����ϢϢ��ء�����ش�������ˮ�йص����⣮



 ���͡�
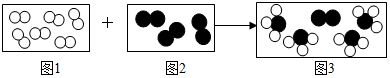
���͡� ���ֱ�������ֲ�ͬ�ĵ��ʷ���A2��B2��������һ���������ܷ�����ѧ��Ӧ���䷴Ӧ����ʾ��ͼ��ͼ��
���ֱ�������ֲ�ͬ�ĵ��ʷ���A2��B2��������һ���������ܷ�����ѧ��Ӧ���䷴Ӧ����ʾ��ͼ��ͼ��