��Ŀ����
 ��2013?�ٳ���ģ�⣩�ס�������ʵ��С��ֱ���С��ⶨNa2CO3��NaCl�������Na2CO3��������ʵ�飮
��2013?�ٳ���ģ�⣩�ס�������ʵ��С��ֱ���С��ⶨNa2CO3��NaCl�������Na2CO3��������ʵ�飮��1�����飺����������
��һ��������Ʒ�ܽ��������CaCl2��Ȼ�����ó������ˡ�ϴ�ӡ���ɡ�������������Ӧ�Ļ�ѧ����ʽΪ
Na2CO3+CaCl2=CaCO3��+2NaCl
Na2CO3+CaCl2=CaCO3��+2NaCl
����2�����飺���������
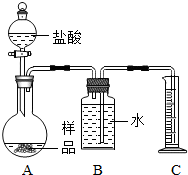
��һ��������Ʒ���������ᷴӦ������ͼװ�òⶨ������CO2�������������ô�װ�òⶨ��ʵ������ȷ��ԭ����
���ڶ�����̼������ˮ����װ���л��в��ֶ�����̼����ˮ����ʹ������岻ȷ�����ʵ������ȷ
���ڶ�����̼������ˮ����װ���л��в��ֶ�����̼����ˮ����ʹ������岻ȷ�����ʵ������ȷ
����3���������һ����ס������������ͬ��ʵ�鷽�����ⶨ�������Na2CO3�ĺ�����
������
��һ��Ũ������ζ�һ�����Ļ����
��һ��Ũ������ζ�һ�����Ļ����
���õ�����Ҫ�Լ���
һ��Ũ�ȵ����ᡢ��̪
һ��Ũ�ȵ����ᡢ��̪
������2�֣����õ��IJ���������
��ͷ�ιܡ��ձ�
��ͷ�ιܡ��ձ�
������2�������������������е�֪ʶ���з�����̼�����������ᷴӦ���ɶ�����̼���壬�����Ȼ��Ʒ�Ӧ����̼��Ƴ�����������̼��������ˮ�����壬�ݴ˽�ɣ�
����⣺��1��̼���������Ȼ��Ʒ�Ӧ����̼��Ƴ������Ȼ��ƣ����Na2CO3+CaCl2=CaCO3��+2NaCl��
��2�����òⶨ���ɵĶ�����̼����������ķ��������ڶ�����̼��������ˮ�����壬��ʹ��õ�����������ȷ����ɽ����ȷ��������ڶ�����̼������ˮ����װ���л��в��ֶ�����̼����ˮ����ʹ������岻ȷ�����ʵ������ȷ��
��3������̼�����������ᷴӦ���ɶ�����̼��������ʹ�ø������ĵ�������������м���ķ�����ʹ�ô˷���ʱҪ֪���������������������̼���Ƶ�ˮ��Һ�ʼ��ԣ���������Һ�м��������ķ�̪��Һ�������жϷ�Ӧ�Ľ��У����õ����������ձ��ͽ�ͷ���أ������һ��Ũ������ζ�һ�����Ļ���һ��Ũ�ȵ����ᡢ��̪����ͷ�ιܡ��ձ���
��2�����òⶨ���ɵĶ�����̼����������ķ��������ڶ�����̼��������ˮ�����壬��ʹ��õ�����������ȷ����ɽ����ȷ��������ڶ�����̼������ˮ����װ���л��в��ֶ�����̼����ˮ����ʹ������岻ȷ�����ʵ������ȷ��
��3������̼�����������ᷴӦ���ɶ�����̼��������ʹ�ø������ĵ�������������м���ķ�����ʹ�ô˷���ʱҪ֪���������������������̼���Ƶ�ˮ��Һ�ʼ��ԣ���������Һ�м��������ķ�̪��Һ�������жϷ�Ӧ�Ľ��У����õ����������ձ��ͽ�ͷ���أ������һ��Ũ������ζ�һ�����Ļ���һ��Ũ�ȵ����ᡢ��̪����ͷ�ιܡ��ձ���
���������⿼���˻������ij�ɷֺ����IJⶨ����ɴ��⣬�����������ʵ����ʽ��У�

��ϰ��ϵ�д�
�����Ŀ

 ��2013?�ٳ���ģ�⣩��Ԫ�ض��������������������Ҫ���壮
��2013?�ٳ���ģ�⣩��Ԫ�ض��������������������Ҫ���壮