��Ŀ����
7�� ��ѧ��ʦָ��ij��ѧ��ȤС�飬�ⶨ�����ǵ���Ҫ�ɷ�̼��Ƶ��������������ǽ�1��2��������뵽l2g�������У�����������̼����������ͼ��ʾ�������輦�����е������ɷֲ������ᷢ����Ӧ��
��ѧ��ʦָ��ij��ѧ��ȤС�飬�ⶨ�����ǵ���Ҫ�ɷ�̼��Ƶ��������������ǽ�1��2��������뵽l2g�������У�����������̼����������ͼ��ʾ�������輦�����е������ɷֲ������ᷢ����Ӧ����1����ͼ�п��Կ�����12g�����������ᷴӦ�����ɶ�����̼��������4.4g��
��2���Լ��㼦������̼��Ƶ����������Ƕ��٣���д��������̣�������������0.1%��
���� ��1������ͼ���ҳ����ɶ�����̼��������
��2�����ݻ�ѧ����ʽ�Ͷ�����̼�������������μӷ�Ӧ��̼��Ƶ�������Ȼ���������������
��� �⣺��1����ͼ�п��Կ�����12g�����������ᷴӦ�����ɶ�����̼��������4.4g��
��2���輦������̼��Ƶ�����Ϊx
CaCO3+2HCl=CaCl2+H2O+CO2��
100 44
x 4.4g
$\frac{100}{x}$=$\frac{44}{4.4g}$
x=10g
��������̼��Ƶ�����������$\frac{10g}{12g}$��100%=83.3%��
�˴�ΰ����1��4.4��
��2����Ʒ��̼��Ƶ�����������83.3%��
���� ���������������뻯ѧ����ʽ���ۺϼ��㣬����Ҫ��ȷд������ʽ���ٸ���������ϸ����������ϵ��������㣬������⣮

��ϰ��ϵ�д�
�����Ŀ
18����ͼ��ʾʵ�������ȷ���ǣ�������
| A�� |  �㵽Һ�� | B�� |  ��ȡ��� | C�� |  ��ȼ�ƾ��� | D�� |  �μ�Һ�� |
2��ʵ�������ռ�һƿԼ����$\frac{1}{4}$������������������в�����ȷ���ǣ�������
 |  |  |  |
| ����ƿ�й�$\frac{1}{4}$��ˮ | ����ƿ�й�$\frac{3}{4}$��ˮ | �������뵽����ƿ�����$\frac{1}{4}$�� | �������뵽����ƿ�����$\frac{3}{4}$�� |
| A�� | A | B�� | B | C�� | C | D�� | D |
19��������֣���־�ɳǣ������ຣ���������ߵ��ڶ��������У���һ�ָ�Ч����������Ҫ�ɷ�Ϊ���Ⱦ���C3O3N3Cl3���������йظ��Ⱦ���˵����ȷ���ǣ�������
| A�� | ���Ⱦ���12��Ԫ����� | |
| B�� | ���Ⱦ�����Ԫ�ص���������Ϊ30% | |
| C�� | һ�����Ⱦ��������ɸ��Ⱦ���3��̼ԭ�ӡ�3����ԭ�ӡ�3����ԭ�Ӻ�3����ԭ�ӹ��� | |
| D�� | ���Ⱦ���C��O��N��Cl��Ԫ�������ȱ�Ϊ1��1��1��1 |
3��FeCl3����������������ֹѪ����ijʵ����ȤС������FeCl3��ʴ��·ͭ������Һ����Ҫ�ɷ�ΪFeCl2��CuCl2���������Ȼ�������ʵ�飮��ʵ��С��ͬѧͨ���������ϣ������˳��ԣ�
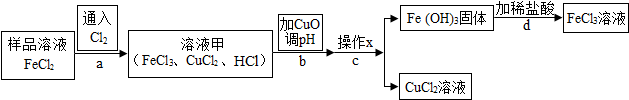
���Ķ����ϡ�
��ͬ����������������ڲ�ͬpH��Χ�ڴ���Һ�г�����������ҵ�����õ�����ҺpH�ķ�����ʹ���������������γ�������Ϲ��˵Ȳ������������ʷ��룮���������������������ͭ��ʼ�����������ȫ��pH
��1������x�ǹ��ˣ�
��2��ʵ���У�����b���� CuO ���� ��Һ���е�HCl��ʹ��ҺpH�������跶Χ����д������������ͭ��Ӧ�Ļ�ѧ����ʽCuO+2HCl=CuCl2+H2O���÷�Ӧ���ڸ��ֽⷴӦ���������Ӧ���ͣ�������Ϊ����b������Һ��pH��3.2��4.7������ֵ��Χ���ȽϺ��ʣ�
��3��ʵ���У�����d��ϡ����ǰ����ȱ��ϴ�ӣ���������ƣ���ʹ��õ��Ȼ�����Һ������
��4��FeCl3��Һ��������Fe�� OH �� 3 �� �����ʣ��ڱ���FeCl3��Һʱ������FeCl3��Һ�м���ij���ᣬ�Է�ֹFeCl3��Һ���ʣ�����Ϊѡ��C������ĸ��ʾ���������
A��ϡ���� B��ϡ���� C��ϡ���� D��ϡ���ᣮ

���Ķ����ϡ�
| Fe��OH��3 | Cu��OH��2 | |
| ��ʼ������pH | 1.9 | 4.7 |
| ������ȫ��pH | 3.2 | 6.7 |
��1������x�ǹ��ˣ�
��2��ʵ���У�����b���� CuO ���� ��Һ���е�HCl��ʹ��ҺpH�������跶Χ����д������������ͭ��Ӧ�Ļ�ѧ����ʽCuO+2HCl=CuCl2+H2O���÷�Ӧ���ڸ��ֽⷴӦ���������Ӧ���ͣ�������Ϊ����b������Һ��pH��3.2��4.7������ֵ��Χ���ȽϺ��ʣ�
��3��ʵ���У�����d��ϡ����ǰ����ȱ��ϴ�ӣ���������ƣ���ʹ��õ��Ȼ�����Һ������
��4��FeCl3��Һ��������Fe�� OH �� 3 �� �����ʣ��ڱ���FeCl3��Һʱ������FeCl3��Һ�м���ij���ᣬ�Է�ֹFeCl3��Һ���ʣ�����Ϊѡ��C������ĸ��ʾ���������
A��ϡ���� B��ϡ���� C��ϡ���� D��ϡ���ᣮ
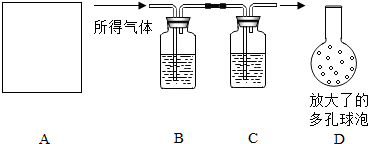 Ϊ�˲ⶨijʯ��ʯ�����Ĵ��ȣ������������ʲ����ᷴӦ����ijͬѧ���������̽���ʵ�飺�������ܽ���������������������NaOH��Һ�������������NaOH��Һ����������������Ĵ��ȣ�ʵ���������ȡ����������Ϊ10g��ʵ��װ����ͼ��ʾ��
Ϊ�˲ⶨijʯ��ʯ�����Ĵ��ȣ������������ʲ����ᷴӦ����ijͬѧ���������̽���ʵ�飺�������ܽ���������������������NaOH��Һ�������������NaOH��Һ����������������Ĵ��ȣ�ʵ���������ȡ����������Ϊ10g��ʵ��װ����ͼ��ʾ��