��Ŀ����
ͨ����ѧѧϰ�����Ѿ�������ʵ������ȡ������йع��ɡ�������ͼ�ش��⣺

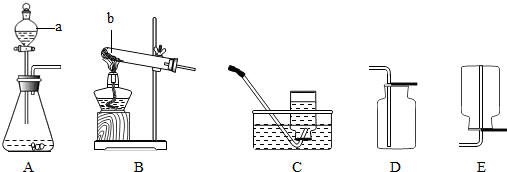
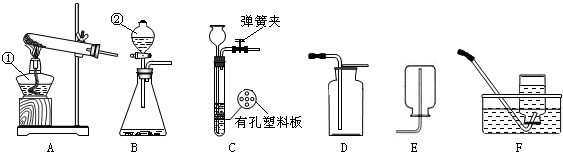
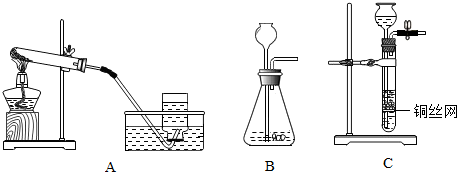
(1)д��ͼ�б�����������ƣ���__________���� ___________��
(2)��֪��������(Na2O2)��һ�ֵ���ɫ�����ĩ�������¿���ˮ��Ӧ�����������ƺ������������ʵ�������ù������ƺ�ˮ��Ӧ����ȡһƿ����������ѡ����װ�á��ռ�װ�÷ֱ���(��дװ����ĸ)________��_______��
(3)��Bװ����ȡ������̼���壬Ӧѡ�õ�Һ��ҩƷ��__________������������Ƿ��ռ����ķ�����__________��
(4)ʵ��������Aװ����ȡ��������Ӧ�Ļ�ѧ����ʽΪ_____________��
(2)��֪��������(Na2O2)��һ�ֵ���ɫ�����ĩ�������¿���ˮ��Ӧ�����������ƺ������������ʵ�������ù������ƺ�ˮ��Ӧ����ȡһƿ����������ѡ����װ�á��ռ�װ�÷ֱ���(��дװ����ĸ)________��_______��
(3)��Bװ����ȡ������̼���壬Ӧѡ�õ�Һ��ҩƷ��__________������������Ƿ��ռ����ķ�����__________��
(4)ʵ��������Aװ����ȡ��������Ӧ�Ļ�ѧ����ʽΪ_____________��
(1)�ƾ��ƣ���Һ©��
(2) B��C����E��
(3)ϡ�����ȼ�ŵ�ľ����������ƿ�ڣ��������Ƿ�Ϩ��
(4) 2KMnO4 K2MnO4 + MnO2 + O2��
K2MnO4 + MnO2 + O2��
(2) B��C����E��
(3)ϡ�����ȼ�ŵ�ľ����������ƿ�ڣ��������Ƿ�Ϩ��
(4) 2KMnO4
 K2MnO4 + MnO2 + O2��
K2MnO4 + MnO2 + O2�� 
��ϰ��ϵ�д�
�����Ŀ
 ͨ����ѧѧϰ�����Ѿ�������ʵ������ȡ�����һЩ���ɣ�������ͼ�ش����⣺
ͨ����ѧѧϰ�����Ѿ�������ʵ������ȡ�����һЩ���ɣ�������ͼ�ش����⣺