��Ŀ����
����̼�����Ŀǰ����Ĺ�ͬ���⣬��������Ⱦ��Ҳ��Ϊ���ͬ��Ŀ�꣮�±��Ǽ���ȼ���ڳ����µ�״̬��1molȼ����ȫȼ��ʱ�ų���������
| ��� | ̿�� | һ����̼ | ������̼ | ���� | �Ҵ� |
| ״̬ | ��̬ | ��̬ | ��̬ | ��̬ | Һ̬ |
| ������kJ�� | 392.8 | 282.6 | 285.8 | 890.3 | 1367 |
���Ҵ���C2H5OH����һ�����ʵ�Һ̬ȼ�ϣ���ȼ�յĻ�ѧ����ʽ��________��
�۽�������ѧ�������о���CO2����ת��Ϊ���飨CH4�����״���CH3OH�������ᣨCH3COOH���Ȼ���ԭ�ϣ���Щ����ԭ�϶�����________�����л�����������������________��Ԫ����ɣ�
CH4 CO C2H5OH+3O2 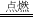 2CO2+3H2O�� ��� 3
2CO2+3H2O�� ��� 3
��������1����һ����̼�����������������ȼ���У�lmol����ȼ�շų���������ࣻ����CO�о綾�ĽǶȷ�����
��2�����ݷ�Ӧ��������P�������غ㶨�ɿ�����д��ѧ����ʽ��
��3�����л��������ĸ�����з�����������Ļ�ѧʽ�ɿ��������Ԫ�ص����࣮
��𣺣�1���������Ƕȷ�����Ŀǰ���ʺϼ�ͥʹ�õ���������ȼ���Ǽ��飮����CO�о綾���ʺ���Ӧ����Щ��
��2����֪�Ҵ�ȼ�յIJ���Ϊ��CO2��H2O����Ӧ����ʽΪ��C2H5OH+3O2 2CO2+3H2O��
2CO2+3H2O��
��3������̼Ԫ�صĻ����������л�����飨CH4�����״���CH3OH�������ᣨCH3COOH�������ʶ��Ǻ���̼Ԫ�صĻ���������л��������Ļ�ѧʽ��֪������������Ԫ����ɵģ�
�ʴ�Ϊ����1��CH4��CO����2��C2H5OH+3O2 2CO2+3H2O����3���л��3��
2CO2+3H2O����3����3��
�����������Ҫ����������Ϣ��������������غ㶨�ɵĺ�����л���������ȣ�ֻ���������ܶ���ط��������������ȷ���жϣ�
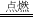 2CO2+3H2O�� ��� 3
2CO2+3H2O�� �л��� 3��������1����һ����̼�����������������ȼ���У�lmol����ȼ�շų���������ࣻ����CO�о綾�ĽǶȷ�����
��2�����ݷ�Ӧ��������P�������غ㶨�ɿ�����д��ѧ����ʽ��
��3�����л��������ĸ�����з�����������Ļ�ѧʽ�ɿ��������Ԫ�ص����࣮
��𣺣�1���������Ƕȷ�����Ŀǰ���ʺϼ�ͥʹ�õ���������ȼ���Ǽ��飮����CO�о綾���ʺ���Ӧ����Щ��
��2����֪�Ҵ�ȼ�յIJ���Ϊ��CO2��H2O����Ӧ����ʽΪ��C2H5OH+3O2
 2CO2+3H2O��
2CO2+3H2O����3������̼Ԫ�صĻ����������л�����飨CH4�����״���CH3OH�������ᣨCH3COOH�������ʶ��Ǻ���̼Ԫ�صĻ���������л��������Ļ�ѧʽ��֪������������Ԫ����ɵģ�
�ʴ�Ϊ����1��CH4��CO����2��C2H5OH+3O2
 2CO2+3H2O����3����3��
2CO2+3H2O����3���л��3�������������Ҫ����������Ϣ��������������غ㶨�ɵĺ�����л���������ȣ�ֻ���������ܶ���ط��������������ȷ���жϣ�

��ϰ��ϵ�д�
�����Ŀ
����̼�����Ŀǰ����Ĺ�ͬ���⣬��������Ⱦ��Ҳ��Ϊ���ͬ��Ŀ�꣮�±��Ǽ���ȼ���ڳ����µ�״̬��1molȼ����ȫȼ��ʱ�ų���������
�ټ�ͥȼ����һ������Ļ���ȼ���ɷֵ��Ż�ʮ����Ҫ���ӷų������ĽǶȷ�����ȼ���� ����ѧʽ���ĺ���������ߣ��ӷ�ֹ�����ж��ĽǶȿ� ����ѧʽ������Ӧ�ü���Щ��
���Ҵ���C2H5OH����һ�����ʵ�Һ̬ȼ�ϣ���ȼ�յĻ�ѧ����ʽ�� ��
�۽�������ѧ�������о���CO2����ת��Ϊ���飨CH4�����״���CH3OH�������ᣨCH3COOH���Ȼ���ԭ�ϣ���Щ����ԭ�϶����� �����л����������������� ��Ԫ����ɣ�
| �� �� | ̿�� | һ����̼ | ������̼ | ���� | �Ҵ� |
| ״̬ | ��̬ | ��̬ | ��̬ | ��̬ | Һ̬ |
| ������kJ�� | 392.8 | 282.6 | 285.8 | 890.3 | 1367 |
���Ҵ���C2H5OH����һ�����ʵ�Һ̬ȼ�ϣ���ȼ�յĻ�ѧ����ʽ��
�۽�������ѧ�������о���CO2����ת��Ϊ���飨CH4�����״���CH3OH�������ᣨCH3COOH���Ȼ���ԭ�ϣ���Щ����ԭ�϶�����
��2011?��������ģ������̼�����Ŀǰ����Ĺ�ͬ���⣬��������Ⱦ��Ҳ��Ϊ���ͬ��Ŀ�꣮�±��Ǽ���ȼ���ڳ����µ�״̬��1molȼ����ȫȼ��ʱ�ų���������
�ټ�ͥȼ����һ������Ļ���ȼ���ɷֵ��Ż�ʮ����Ҫ���ӷų������ĽǶȷ�����ȼ���У���ѧʽ���ĺ���������ߣ��ӷ�ֹ�����ж��ĽǶȿ�����ѧʽ������Ӧ�ü���Щ��
���Ҵ���C2H5OH����һ�����ʵ�Һ̬ȼ�ϣ���ȼ�յĻ�ѧ����ʽ����
�۽�������ѧ�������о���CO2����ת��Ϊ���飨CH4�����״���CH3OH�������ᣨCH3COOH���Ȼ���ԭ�ϣ���Щ����ԭ�϶����ڣ����л�������������������Ԫ����ɣ�
| �� �� | ̿�� | һ����̼ | ������̼ | ���� | �Ҵ� |
| ״̬ | ��̬ | ��̬ | ��̬ | ��̬ | Һ̬ |
| ������kJ�� | 392.8 | 282.6 | 285.8 | 890.3 | 1367 |
���Ҵ���C2H5OH����һ�����ʵ�Һ̬ȼ�ϣ���ȼ�յĻ�ѧ����ʽ����
�۽�������ѧ�������о���CO2����ת��Ϊ���飨CH4�����״���CH3OH�������ᣨCH3COOH���Ȼ���ԭ�ϣ���Щ����ԭ�϶����ڣ����л�������������������Ԫ����ɣ�