��Ŀ����
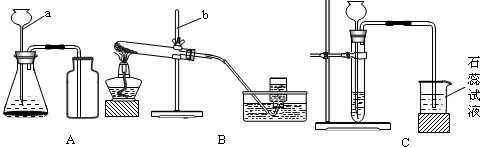
����ΪС��ͬѧ��ʵ������ȡ�����������̼���ʵ�ʵ��װ��ʾ��ͼ��
�Իش�
��1�����������ƣ�a_____________________��b_____________________��
��2�����������Aװ����ȡ����ʱ��һ��ע������______________________________��
Aװ�û�����������ȡ________���壬��ȡ����������ҩƷ��_________��_________��
��3����ͬѧ��ʵ������У���A�в���������ͨ�뵽B�У��۲쵽����ʯ��ˮû�б���ǣ��Է�����ԭ��__________________________________________��
��4����ͬѧҪ��Cƿ�ռ�һƿ��װ��A�в��������壬�������?
__________________________________________________________________________��
��Cƿ�е���Ũ����������Һ��Ѹ�������Ȱ��һ��С����Ľ��������������Թ۲쵽С������____________����ԭ����_____________________�� ��д��Cƿ�з�Ӧ�Ļ�ѧ����ʽ_______________________________________________________________��
��5������̽��ʵ��B��C��������֤������̼����_____________________�����ʷ�����Ӧ��������̼������Щ���ʣ��Ծٳ���������д����֮��ص�һ����;��
���ʣ�_____________________����Ӧ����;��_____________________��
���ʣ�_____________________����Ӧ����;��_____________________��
��1���Թ� ����©��
��2������©���������Һ������ H2��O2 Zn ϡH2SO4
��3���õ�����Ũ�����ʯ��ˮ����
��4����ȼ�ŵ�ľ������Cƿ�ڣ���ľ��Ϩ�������� ���� ƿ��CO2��NaOH��Ӧ��ʹƿ��ѹǿ��С CO2+2NaOH=Na2CO3+H2O
��5���� ��ȼ�� ��֧��ȼ�� ��� ��ˮ��Ӧ ��̼������

| |||||||||||||||||||||||
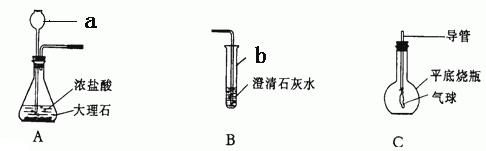
����ΪС��ͬѧ��ʵ������ȡ�����������̼���ʵ�ʵ��װ��ʾ��ͼ��
 |
�Իش�
(1) ���������ƣ�a �� b ��
(2) ���������Aװ����ȡ����ʱ��һ��ע������ ��
Aװ�û�����������ȡ ���壬��ȡ����������ҩƷ�� �� ��
(3)��ͬѧ��ʵ������У���A�в���������ͨ�뵽B�У��۲쵽����ʯ��ˮû�б���ǣ��Է�����ԭ�� ��
(4)��ͬѧҪ��Cƿ�ռ�һƿ��װ��A�в��������壬���������
��
��Cƿ�е���Ũ����������Һ��Ѹ�������Ȱ��һ��С����Ľ��������������Թ۲쵽С������ ����ԭ���� ����д��Cƿ�з�Ӧ�Ļ�ѧ����ʽ ��
��5������̽��ʵ��B��C��������֤������̼���� �����ʷ�����Ӧ��������̼������Щ���ʣ��Ծٳ���������д����֮��ص�һ����;��
���ʣ� �� ��Ӧ����;�� ��
���ʣ� �� ��Ӧ����;�� ��