��Ŀ����
�±���NaOH��Ca(OH)2���ܽ�����ݣ���ش��������⡣

��1���ӱ������ݿ��Ի�õ���Ϣ��__________________��дһ������
��2����80��ʱNaOH�ı�����Һ������20�棬���Կ�����������_______________������20��ʱCa(OH)2�ı�����Һ������Һ���������м���һ����CaO��õ�����Һ������Һ������ʱ��Һ����������������______�ף��������������
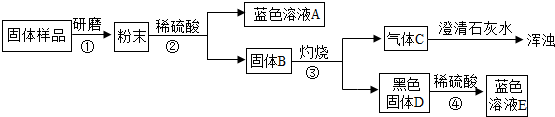
��3��ij��ȤС��Բ��ֱ��ʵ��������ƹ�������ᴿ����������²������̣���ش�
��2����80��ʱNaOH�ı�����Һ������20�棬���Կ�����������_______________������20��ʱCa(OH)2�ı�����Һ������Һ���������м���һ����CaO��õ�����Һ������Һ������ʱ��Һ����������������______�ף��������������
��3��ij��ȤС��Բ��ֱ��ʵ��������ƹ�������ᴿ����������²������̣���ش�

������ڷ�Ӧ�Ļ�ѧ����ʽΪ________________���������Ca(OH)2��Ŀ����___________��
������ҺB�е�������_______��__________��д��ѧʽ����������������ľ�������Ǽ���Ũ����________�����ˡ�
������ҺB�е�������_______��__________��д��ѧʽ����������������ľ�������Ǽ���Ũ����________�����ˡ�
��1��NaOH���ܽ��ԶԶ����Ca(OH)2���ܽ�ȣ���NaOH���ܽ�����¶ȵ����߶�����Ca(OH)2���ܽ�����¶ȵ����߶���С���������������֣�
��2����Һ����ǣ����о�����������<
��3������Na2CO3+Ca(OH)2==CaCO3��+2NaOH������Һ�е�Na2CO3ȫ��ת��ΪNaOH
����NaOH��Ca(OH)2�����½ᾧ
��2����Һ����ǣ����о�����������<
��3������Na2CO3+Ca(OH)2==CaCO3��+2NaOH������Һ�е�Na2CO3ȫ��ת��ΪNaOH
����NaOH��Ca(OH)2�����½ᾧ

��ϰ��ϵ�д�
 ÿ��10���ӿ�����������������ϵ�д�
ÿ��10���ӿ�����������������ϵ�д�
�����Ŀ
�±���NaOH��Ca��OH��2���ܽ�����ݣ���ش��������⣮
| �¶ȣ��棩 | 0 | 20 | 40 | 60 | 80 | 100 | |
| �ܽ�� ��g�� | NaOH | 31 | 91 | 111 | 129 | 313 | 336 |
| Ca��OH��2 | 0.19 | 0.17 | 0.14 | 0.12 | 0.09 | 0.08 | |

��2��ij��ȤС��Բ��ֱ��ʵ��������ƹ�������ᴿ���������ͼ�������̣���ش�

�ٲ���ڷ�Ӧ�Ļ�ѧ����ʽΪ________��
����ҺB�е����������֣����Ƿֱ���________��д��ѧʽ����
�۲�����������ľ�������Ǽ���Ũ����________�����ˣ�