��Ŀ����
a��2005��5��22������11ʱ08�֣��й���ɽ�����ӳɹ�������嶥�����Ǽ�1975����ҹ��ٴζ����������߶Ƚ��о�ȷ�������⻯�ƣ�CaH2�������ǵ�ɽ��Ա���õ���Դ�ṩ����������ˮ��Ӧ�����������ƺ�������CaH2+2H2O�TCa��OH��2+2H2��������ȼ��֮�裮����84g�⻯��������ˮ��Ӧ�������ɶ��ٿ�������
b��Ϊ�˲ⶨijͭп�Ͻ𣨼�ͭп������п������������ijͬѧ���øúϽ���ϡ����
��Ӧ������������ʵ�飬������ص�ʵ�����ݼ�¼���£�ʵ���е������Բ��ƣ���
| ��һ�� | �ڶ��� | ������ | |
| ��ȡ�Ͻ�������Mg | 25 | 25 | 50 |
| ����ϡ����������Mg | 120 | 160 | 100 |
| ���������������Mg | 0.4 | 0.4 | 0.4 |
��1���Լ����ͭп�Ͻ���п������������
��2�����ϱ����ݷ���������ȡ�Ͻ�������ϡ�����������Ϊ______ʱ�������Ͻ��е�п��ϡ�����е�����ǡ����ȫ��Ӧ��
��3�����ļ��η�Ӧ��������ʣ�ࣿ______��
�⣺a����84g�������ƺ�����ˮ��Ӧ��������������Ϊxg��
CaH2+2H2O=Ca��OH��2+2H2��
42 4
84g x
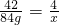
���x=8g��
��84g��������������ˮ��Ӧ��������������Ϊ8g��
b����1����ʵ�����ݿ�֪��25g�Ͻ��������ᷴӦ�������0.4g������
��25g�Ͻ���п������Ϊyg����
Zn+H2SO4�TZnSO4+H2��
65 2
y 0.4g
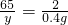
���y=13g��
��ͭп�Ͻ���п����������Ϊ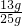 ��100%=52%��
��100%=52%��
��ͭп�Ͻ���п����������Ϊ52%��
��2����ʵ��һ��ʵ���������ݿ�֪��25g�Ͻ��100g���ᶼ���ڷ�Ӧ�����ɵ�������������
��25g�Ͻ���100g����ǡ����ȫ��Ӧ������������Ϊ25g��100g=1��4���ʴ�Ϊ��1��4��
��3����25g�Ͻ���100g����ǡ����ȫ��Ӧ����25g�Ͻ���120g���ᡢ160g���ᷴӦʱ���ᶼ������
����һ�κ͵ڶ������ᶼ��ʣ�࣬�ʴ�Ϊ����һ�Ρ��ڶ��Σ�
������a�������⻯�ƺ�ˮ��Ӧ�����������ƺ������ķ�Ӧ����ʽ����ˮ���������⻯�Ƶ��������뻯ѧ��Ӧ����ʽ��������������������
b�����ݱ����е�ʵ�����ݿ�֪��25g�Ͻ��������ᷴӦ�������0.4g��������������������������п�����ᷴӦ�ķ���ʽ������п����������������Ͻ���п�������������ɵ����ε�ʵ�����ݿ�֪�Ͻ��������Ӧǡ�÷�ӦҲ����0.4g��������25g�Ͻ���100g����ǡ����ȫ��Ӧ�����
���������⿼�黯ѧ��Ӧ����ʽ�ļ��㣬ѧ��Ӧע���������ʵ��������뷽��ʽ�����㣬ͨ�����Ӧѧ��ʵ�����ݵķ�����Ӧ�ã���ȷ�Ͻ�����ǡ����ȫ��Ӧ�����ǽ��Ĺؼ���
CaH2+2H2O=Ca��OH��2+2H2��
42 4
84g x
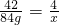
���x=8g��
��84g��������������ˮ��Ӧ��������������Ϊ8g��
b����1����ʵ�����ݿ�֪��25g�Ͻ��������ᷴӦ�������0.4g������
��25g�Ͻ���п������Ϊyg����
Zn+H2SO4�TZnSO4+H2��
65 2
y 0.4g
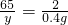
���y=13g��
��ͭп�Ͻ���п����������Ϊ
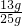 ��100%=52%��
��100%=52%����ͭп�Ͻ���п����������Ϊ52%��
��2����ʵ��һ��ʵ���������ݿ�֪��25g�Ͻ��100g���ᶼ���ڷ�Ӧ�����ɵ�������������
��25g�Ͻ���100g����ǡ����ȫ��Ӧ������������Ϊ25g��100g=1��4���ʴ�Ϊ��1��4��
��3����25g�Ͻ���100g����ǡ����ȫ��Ӧ����25g�Ͻ���120g���ᡢ160g���ᷴӦʱ���ᶼ������
����һ�κ͵ڶ������ᶼ��ʣ�࣬�ʴ�Ϊ����һ�Ρ��ڶ��Σ�
������a�������⻯�ƺ�ˮ��Ӧ�����������ƺ������ķ�Ӧ����ʽ����ˮ���������⻯�Ƶ��������뻯ѧ��Ӧ����ʽ��������������������
b�����ݱ����е�ʵ�����ݿ�֪��25g�Ͻ��������ᷴӦ�������0.4g��������������������������п�����ᷴӦ�ķ���ʽ������п����������������Ͻ���п�������������ɵ����ε�ʵ�����ݿ�֪�Ͻ��������Ӧǡ�÷�ӦҲ����0.4g��������25g�Ͻ���100g����ǡ����ȫ��Ӧ�����
���������⿼�黯ѧ��Ӧ����ʽ�ļ��㣬ѧ��Ӧע���������ʵ��������뷽��ʽ�����㣬ͨ�����Ӧѧ��ʵ�����ݵķ�����Ӧ�ã���ȷ�Ͻ�����ǡ����ȫ��Ӧ�����ǽ��Ĺؼ���

��ϰ��ϵ�д�
�����Ŀ
��������a��b��С�⣬������ѡһ���𣬲������ⶼ�⣮�����ⶼ�⣬����aС��Ʒ֣�
a��2005��5��22������11ʱ08�֣��й���ɽ�����ӳɹ�������嶥�����Ǽ�1975����ҹ��ٴζ����������߶Ƚ��о�ȷ�������⻯�ƣ�CaH2�������ǵ�ɽ��Ա���õ���Դ�ṩ����������ˮ��Ӧ�����������ƺ�������CaH2+2H2O�TCa��OH��2+2H2��������ȼ��֮�裮����84g�⻯��������ˮ��Ӧ�������ɶ��ٿ�������
b��Ϊ�˲ⶨijͭп�Ͻ𣨼�ͭп������п������������ijͬѧ���øúϽ���ϡ����
��Ӧ������������ʵ�飬������ص�ʵ�����ݼ�¼���£�ʵ���е������Բ��ƣ���
��1���Լ����ͭп�Ͻ���п������������
��2�����ϱ����ݷ���������ȡ�Ͻ�������ϡ�����������Ϊ ʱ�������Ͻ��е�п��ϡ�����е�����ǡ����ȫ��Ӧ��
��3�����ļ��η�Ӧ��������ʣ�ࣿ ��
a��2005��5��22������11ʱ08�֣��й���ɽ�����ӳɹ�������嶥�����Ǽ�1975����ҹ��ٴζ����������߶Ƚ��о�ȷ�������⻯�ƣ�CaH2�������ǵ�ɽ��Ա���õ���Դ�ṩ����������ˮ��Ӧ�����������ƺ�������CaH2+2H2O�TCa��OH��2+2H2��������ȼ��֮�裮����84g�⻯��������ˮ��Ӧ�������ɶ��ٿ�������
b��Ϊ�˲ⶨijͭп�Ͻ𣨼�ͭп������п������������ijͬѧ���øúϽ���ϡ����
��Ӧ������������ʵ�飬������ص�ʵ�����ݼ�¼���£�ʵ���е������Բ��ƣ���
| ��һ�� | �ڶ��� | ������ | |
| ��ȡ�Ͻ�������Mg | 25 | 25 | 50 |
| ����ϡ����������Mg | 120 | 160 | 100 |
| ���������������Mg | 0.4 | 0.4 | 0.4 |
��2�����ϱ����ݷ���������ȡ�Ͻ�������ϡ�����������Ϊ
��3�����ļ��η�Ӧ��������ʣ�ࣿ
��������a��b��С�⣬������ѡһ���𣬲������ⶼ�⣮�����ⶼ�⣬����aС��Ʒ֣�
a��2005��5��22������11ʱ08�֣��й���ɽ�����ӳɹ�������嶥�����Ǽ�1975����ҹ��ٴζ����������߶Ƚ��о�ȷ�������⻯�ƣ�CaH2�������ǵ�ɽ��Ա���õ���Դ�ṩ����������ˮ��Ӧ�����������ƺ�������CaH2+2H2O�TCa��OH��2+2H2��������ȼ��֮�裮����84g�⻯��������ˮ��Ӧ�������ɶ��ٿ�������
b��Ϊ�˲ⶨijͭп�Ͻ𣨼�ͭп������п������������ijͬѧ���øúϽ���ϡ����
��Ӧ������������ʵ�飬������ص�ʵ�����ݼ�¼���£�ʵ���е������Բ��ƣ���
��1���Լ����ͭп�Ͻ���п������������
��2�����ϱ����ݷ���������ȡ�Ͻ�������ϡ�����������Ϊ______ʱ�������Ͻ��е�п��ϡ�����е�����ǡ����ȫ��Ӧ��
��3�����ļ��η�Ӧ��������ʣ�ࣿ______��
a��2005��5��22������11ʱ08�֣��й���ɽ�����ӳɹ�������嶥�����Ǽ�1975����ҹ��ٴζ����������߶Ƚ��о�ȷ�������⻯�ƣ�CaH2�������ǵ�ɽ��Ա���õ���Դ�ṩ����������ˮ��Ӧ�����������ƺ�������CaH2+2H2O�TCa��OH��2+2H2��������ȼ��֮�裮����84g�⻯��������ˮ��Ӧ�������ɶ��ٿ�������
b��Ϊ�˲ⶨijͭп�Ͻ𣨼�ͭп������п������������ijͬѧ���øúϽ���ϡ����
��Ӧ������������ʵ�飬������ص�ʵ�����ݼ�¼���£�ʵ���е������Բ��ƣ���
| ��һ�� | �ڶ��� | ������ | |
| ��ȡ�Ͻ�������Mg | 25 | 25 | 50 |
| ����ϡ����������Mg | 120 | 160 | 100 |
| ���������������Mg | 0.4 | 0.4 | 0.4 |
��2�����ϱ����ݷ���������ȡ�Ͻ�������ϡ�����������Ϊ______ʱ�������Ͻ��е�п��ϡ�����е�����ǡ����ȫ��Ӧ��
��3�����ļ��η�Ӧ��������ʣ�ࣿ______��