��Ŀ����
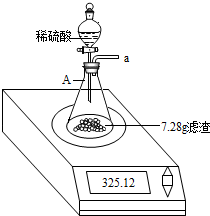
��ѧ��ȤС���ijʯ��ʯ��Ʒ��̼��Ƶĺ�������ʵ��̽������С��ȡ��4g��Ʒ���ⶨ��ʯ��ʯ�Ĵ��ȣ���δ֪��������������ϡ����40g��4�μ��룮ÿ�γ�ַ�Ӧ�����ˡ�����Ȳ���������������������������ˡ�����Ȳ������̣��������ʵ�����������ģ�ʯ��ʯ�е����ʲ������ᷴӦ��Ҳ������ˮ����ʵ�������±���
����㣺��1����ʯ��ʯ��Ʒ��̼��Ƶ����������Ƕ��٣�
��2�����õ�ϡ�����У����ʵ����������Ƕ��٣�
��3��4gʯ��ʯ��Ʒ��������ϡ���ᷴӦ�����ɶ�����̼���ٿˣ�
| ϡ��������� |
��һ�μ���10g |
�ڶ��μ���10g |
�������10g |
���Ĵμ���10g |
| ʣ���������� | 3.0g | 2.0g | 1.0g | 0.4g |
��2�����õ�ϡ�����У����ʵ����������Ƕ��٣�
��3��4gʯ��ʯ��Ʒ��������ϡ���ᷴӦ�����ɶ�����̼���ٿˣ�
��1��ʯ��ʯ��Ʒ��CaCO3����������Ϊ��
��100%=90%
��ʯ��ʯ��Ʒ��̼��Ƶ���������Ϊ90%
��2�����һ��ʵ���У��������10g���������ʵ�����ΪX
CaCO3+2HCl�TCaCl2+H2O+CO2��
100 73
1g X
=
x=0.73g
ϡ���������ʵ���������=
��100%=7.3%
��ϡ���������ʵ���������Ϊ7.3%
��3�����ʵ�����ɶ�����̼������ΪY
CaCO3+2HCl�TCaCl2+H2O+CO2��
100 44
3.6g Y
=
y=1.6g
�����ɶ�����̼1.6g��
| 4g-0.4g |
| 4g |
��ʯ��ʯ��Ʒ��̼��Ƶ���������Ϊ90%
��2�����һ��ʵ���У��������10g���������ʵ�����ΪX
CaCO3+2HCl�TCaCl2+H2O+CO2��
100 73
1g X
| 100 |
| 1g |
| 73 |
| x |
x=0.73g
ϡ���������ʵ���������=
| 0.73g |
| 10g |
��ϡ���������ʵ���������Ϊ7.3%
��3�����ʵ�����ɶ�����̼������ΪY
CaCO3+2HCl�TCaCl2+H2O+CO2��
100 44
3.6g Y
| 100 |
| 3.6g |
| 44 |
| y |
y=1.6g
�����ɶ�����̼1.6g��

��ϰ��ϵ�д�
 ������ÿ�ʱ�Ż���ҵϵ�д�
������ÿ�ʱ�Ż���ҵϵ�д�
�����Ŀ
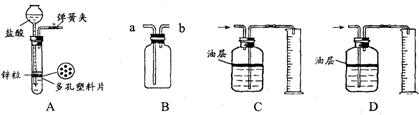
 һ��ѧ��ȤС���ij�±���װ���еġ����������ܺ��棬���ǹ۲쵽�������������װ��ע�ijɷ�Ϊ���ۡ�����̿���Ȼ��ƣ����ֻҺ�ɫ�Ĺ����л���������������ɫ��ĩ��
һ��ѧ��ȤС���ij�±���װ���еġ����������ܺ��棬���ǹ۲쵽�������������װ��ע�ijɷ�Ϊ���ۡ�����̿���Ȼ��ƣ����ֻҺ�ɫ�Ĺ����л���������������ɫ��ĩ��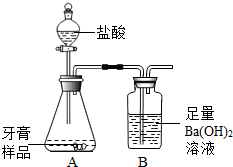 ��2013?�����ж�ģ����ѧ��ȤС���ijƷ��������̼��ƺ�����������̽����������Ħ������Ҫ��̼��ơ�����������ɣ������ɷ���������ʱ���������ɣ���������ͼ��ʾװ�ã�ͼ�мг�������ȥ������ʵ�飬��ַ�Ӧ�ⶨB�����ɵ�BaCO3������������ȷ��̼��Ƶ�������������ش��������⣮
��2013?�����ж�ģ����ѧ��ȤС���ijƷ��������̼��ƺ�����������̽����������Ħ������Ҫ��̼��ơ�����������ɣ������ɷ���������ʱ���������ɣ���������ͼ��ʾװ�ã�ͼ�мг�������ȥ������ʵ�飬��ַ�Ӧ�ⶨB�����ɵ�BaCO3������������ȷ��̼��Ƶ�������������ش��������⣮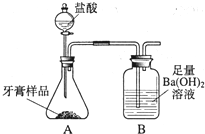 ��ѧ��ȤС���ijƷ��������̼��ƺ�����������̽����������Ħ������Ҫ��̼��ơ�����������ɣ������ɷ���������ʱ���������ɣ���������ͼ��ʾװ�ã�ͼ�мг�������ȥ������ʵ�飬��ַ�Ӧ�ⶨB�����ɵ�BaCO3������������ȷ��̼��Ƶ�������������ش��������⣮
��ѧ��ȤС���ijƷ��������̼��ƺ�����������̽����������Ħ������Ҫ��̼��ơ�����������ɣ������ɷ���������ʱ���������ɣ���������ͼ��ʾװ�ã�ͼ�мг�������ȥ������ʵ�飬��ַ�Ӧ�ⶨB�����ɵ�BaCO3������������ȷ��̼��Ƶ�������������ش��������⣮ һ��ѧ��ȤС���ij�±���װ���еġ����������ܺ��棬���ǹ۲쵽�������������װ��ע�ijɷ�Ϊ���ۡ�����̿���Ȼ��ƣ����ֻҺ�ɫ�Ĺ����л���������������ɫ��ĩ��
һ��ѧ��ȤС���ij�±���װ���еġ����������ܺ��棬���ǹ۲쵽�������������װ��ע�ijɷ�Ϊ���ۡ�����̿���Ȼ��ƣ����ֻҺ�ɫ�Ĺ����л���������������ɫ��ĩ��