��Ŀ����
��һƿ���壬������CO��CO2��H2O��H2�е�һ�ֻ�����ɣ�Ϊ�˷���������ijɷ֣�ȡ�������������ʵ�飬ʵ�鷽��������£����������ϣ���ɫ��ˮ����ͭ��ĩ��ˮ����ɫ����
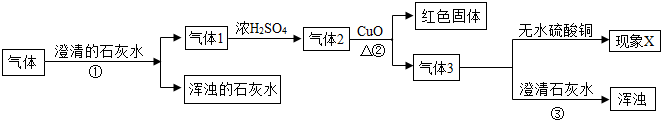
��1����������һ�����е���
��2��������XΪ����ˮ����ͭ����ɫ����������������
��3������һ�������ķ�Ӧ����ʽ��
��4���٢۷����ķ�Ӧ��ͬ���䷴Ӧ�ķ���ʽ��
��5����������ʵ�鷽������ȷ��H2O�Ĵ�������ĵķ�����

��1����������һ�����е���
CO2
CO2
����2��������XΪ����ˮ����ͭ����ɫ����������������
H2
H2
��������XΪ����ˮ����ͭ����ɫ����������������H2
H2
����3������һ�������ķ�Ӧ����ʽ��
CO+CuO
Cu+CO2
| ||
CO+CuO
Cu+CO2
��
| ||
��4���٢۷����ķ�Ӧ��ͬ���䷴Ӧ�ķ���ʽ��
CO2+Ca��OH��2=CaCO3��+H2O
CO2+Ca��OH��2=CaCO3��+H2O
����5����������ʵ�鷽������ȷ��H2O�Ĵ�������ĵķ�����
�Ƚ��������ͨ����ˮ����ͭ�У�������ˮ����ͭ����ɫ������˵����������к���H2O��������ˮ����ͭ����ɫ������˵����������в�����H2O��
�Ƚ��������ͨ����ˮ����ͭ�У�������ˮ����ͭ����ɫ������˵����������к���H2O��������ˮ����ͭ����ɫ������˵����������в�����H2O��
��������������̼������ʹ�����ʯ��ˮ����ǣ�Ũ�����ڸ�ʵ�����Ǹ������������һ����̼�����л�ԭ�ԣ���ˮ����ͭ��ˮ����ɫ��
����⣺��1�����������ʹ����ʯ��ˮ�ٱ���ǣ�˵���û��������һ������CO2��
���CO2��
��2������1ͨ��Ũ���������2�п϶�û��H2O�ˣ�������X�С���ˮ����ͭ����ɫ����˵������3�к���H2O������CuO��Ӧ�Ļ�������п϶��к��С�H��ԭ�ӵĻ�ԭ������
H2��������X�С���ˮ����ͭ����ɫ����˵������3�в�����H2O������CuO��Ӧ�Ļ�������п϶����С�H��ԭ�ӵĻ�ԭ������H2��
���H2��H2��
��3��������3ͨ������ʯ��ˮ�������ʯ��ˮ����ǡ���һ����˵������3��һ������CO2��������CuO��Ӧ�Ļ�������п϶��к��С�C��ԭ�ӵĻ�ԭ������CO�����ԣ���Ӧ����һ�������ķ�Ӧ����ʽ�ǣ�CO+CuO
Cu+CO2
���CO+CuO
Cu+CO2��
��4����ʹ����ʯ��ˮ����ǵ�ԭ���Ƕ�����̼������������Һ������Ӧ���ɲ�����ˮ��̼��ƣ��䷴Ӧ����ʽΪ��CO2+Ca��OH��2=CaCO3��+H2O��
���CO2+Ca��OH��2�TCaCO3��+H2O��
��5������ֱ�����á���ˮ����ͭ��ˮ������������������ˮ�Ĵ������
����Ƚ��������ͨ����ˮ����ͭ�У�������ˮ����ͭ����ɫ������˵����������к���H2O��������ˮ����ͭ����ɫ������˵����������в�����H2O��
���CO2��
��2������1ͨ��Ũ���������2�п϶�û��H2O�ˣ�������X�С���ˮ����ͭ����ɫ����˵������3�к���H2O������CuO��Ӧ�Ļ�������п϶��к��С�H��ԭ�ӵĻ�ԭ������
H2��������X�С���ˮ����ͭ����ɫ����˵������3�в�����H2O������CuO��Ӧ�Ļ�������п϶����С�H��ԭ�ӵĻ�ԭ������H2��
���H2��H2��
��3��������3ͨ������ʯ��ˮ�������ʯ��ˮ����ǡ���һ����˵������3��һ������CO2��������CuO��Ӧ�Ļ�������п϶��к��С�C��ԭ�ӵĻ�ԭ������CO�����ԣ���Ӧ����һ�������ķ�Ӧ����ʽ�ǣ�CO+CuO
| ||
���CO+CuO
| ||
��4����ʹ����ʯ��ˮ����ǵ�ԭ���Ƕ�����̼������������Һ������Ӧ���ɲ�����ˮ��̼��ƣ��䷴Ӧ����ʽΪ��CO2+Ca��OH��2=CaCO3��+H2O��
���CO2+Ca��OH��2�TCaCO3��+H2O��
��5������ֱ�����á���ˮ����ͭ��ˮ������������������ˮ�Ĵ������
����Ƚ��������ͨ����ˮ����ͭ�У�������ˮ����ͭ����ɫ������˵����������к���H2O��������ˮ����ͭ����ɫ������˵����������в�����H2O��
������ͨ������ѧ������ǻ�ѧ�г��õ�һ�ּ������ʵķ�������ͨ����ѧʵ�齫���ֲ�ͬ���Ե�������������

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ