��Ŀ����
��ȤС���ͬѧΪ�ⶨijһ��ͭ�Ͻ��к�����������������6g�úϽ��ĩ��Ʒ������������������Ϊ10%������ͭ��Һ160g�У�����ǡ����ȫ��Ӧ��ͬʱΪ�˳��������Դ�����Է�Ӧ������ʽ��л��մ�������������ͼʽ���㣺

��1���úϽ���Ʒ�к�������������������������ȷ��0.1%��
��2�����ù���ͭ������aΪ���ٿˣ�
��3��������Һ�м�����ٿ�ˮ���ܵõ�5%������������Һ������ȱ�����ܵ�Ӫ��Һ������2����3���м�������ȷ��0.1g��

��1���úϽ���Ʒ�к�������������������������ȷ��0.1%��
��2�����ù���ͭ������aΪ���ٿˣ�
��3��������Һ�м�����ٿ�ˮ���ܵõ�5%������������Һ������ȱ�����ܵ�Ӫ��Һ������2����3���м�������ȷ��0.1g��
���㣺���ݻ�ѧ��Ӧ����ʽ�ļ���,��ˮϡ�ı�Ũ�ȵķ���
ר�⣺�ۺϼ��㣨ͼ���͡������͡��龰�ͼ����⣩
��������1����������ͭ����������μӷ�Ӧ���������Ӷ�������������������н��
��2��������������ͭ�ķ���ʽ��������ɵ�ͭ�ټ�����Ʒ�е�ͭ����a��ֵ���н��
��3�������������Һ��������������������ϡ����Һʱ��������������
��2��������������ͭ�ķ���ʽ��������ɵ�ͭ�ټ�����Ʒ�е�ͭ����a��ֵ���н��
��3�������������Һ��������������������ϡ����Һʱ��������������
����⣺��1���裺��Ʒ����������Ϊx������ͭ������Ϊy��������������������Ϊz��
���������=160g��10%=16g
Fe+CuSO4 =FeSO4 +Cu
56 160 152 64
x 16g z y
=
��
=
��
=
x=5.6g��y=6.4g��z=15.2g
��1���úϽ���Ʒ�к���������������
��100%=93.3%��
��2�����ù���ͭ������aΪ��6.4g+��6g-5.6g��=6.8g��
��3���裬�õ�5%������������Һ����������Һ�м���ˮ������Ϊm��
��100%=5%
m=144.8g
�𣺣�1���úϽ���Ʒ�к�������������93.3%��
��2�����ù���ͭ������aΪ6.8g��
��3���õ�5%������������Һ����������Һ�м���ˮ������Ϊ144.8g��
���������=160g��10%=16g
Fe+CuSO4 =FeSO4 +Cu
56 160 152 64
x 16g z y
| 56 |
| x |
| 160 |
| 16g |
| 160 |
| 16g |
| 64 |
| y |
| 160 |
| 16g |
| 152 |
| z |
x=5.6g��y=6.4g��z=15.2g
��1���úϽ���Ʒ�к���������������
| 5.6g |
| 6g |
��2�����ù���ͭ������aΪ��6.4g+��6g-5.6g��=6.8g��
��3���裬�õ�5%������������Һ����������Һ�м���ˮ������Ϊm��
| 15.2g |
| 5.6g+160g-6.4g+m |
m=144.8g
�𣺣�1���úϽ���Ʒ�к�������������93.3%��
��2�����ù���ͭ������aΪ6.8g��
��3���õ�5%������������Һ����������Һ�м���ˮ������Ϊ144.8g��
���������ջ�ѧ����ʽ�ļ����ʽ�淶�ԣ�������ѵ��ǣ�3���ļ��㣬һ��Ҫ�������ʵ�������

��ϰ��ϵ�д�
�����Ŀ
 ���Ͽ����Ƚ���ֹⷳ�ʣ���ͼ�г������ϵ�Ӫ���ɷ֣�
���Ͽ����Ƚ���ֹⷳ�ʣ���ͼ�г������ϵ�Ӫ���ɷ֣�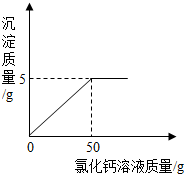 ��ҵ����̼������Һ��ʯ��ˮ��Ӧ���ռ����̼������Һ��ʯ��ˮ�Ƿ�ǡ����ȫ��Ӧ��ij��ѧ��ȤС���ͬѧ���������й��ˣ�������Һ��������̽����
��ҵ����̼������Һ��ʯ��ˮ��Ӧ���ռ����̼������Һ��ʯ��ˮ�Ƿ�ǡ����ȫ��Ӧ��ij��ѧ��ȤС���ͬѧ���������й��ˣ�������Һ��������̽����
 2014��4��2�ա�4�շ������廪ѧ��PX����������ս���������ڰٶȰٿ���������PX��Ϊ���Ͷ��������ȷ����������˽�ʾ��������ʵ�Ŀ�ѧ̬�ȣ�PX�ǡ��Զ��ױ���������ɺͽṹ��ͼ������������£�
2014��4��2�ա�4�շ������廪ѧ��PX����������ս���������ڰٶȰٿ���������PX��Ϊ���Ͷ��������ȷ����������˽�ʾ��������ʵ�Ŀ�ѧ̬�ȣ�PX�ǡ��Զ��ױ���������ɺͽṹ��ͼ������������£�