��Ŀ����
�ݱ������Ĵ����ǿ�ѧ�����ڽ���һ��ʵ�飬���ǽ�ȼú�糧�����Ķ�����̼������ͨ���ܵ�ͨ�����2km����Դﵽ���ٶ�����̼��������ŷŵ�Ŀ�ģ�
��1�����������ļ��ٶ�����̼�Ĵ�ʩ��
��2��ijУ��ѧС���ͬѧΪ̽��������й����ŷŶ�����̼�Ի�����Ӱ�죬��������ʵ�飮ʵ������У����ǽ�����װ��ͬʱ������ͬ�Ļ�����������ͬ��ʱ�䣨�������еĺ�ɫҺ�岻������еijɷַ�����Ӧ��

��ʵ������пɹ۲쵽��������ʲô��
�ڷ�������ʵ������IJ���ɵõ��Ľ�����ʲô��
��3��2007��9��1�գ����ҷ���ί����������ʮ�߸�������ȫ����ʽ���𡰽��ܼ���ȫ���ж�����Ϊ�����Դ��ȱ�ͻ�����Ⱦ���⣬�д��������������õ�����Դ�� ��д�����ּ��ɣ���
��4��ȡ2gʯ��ʯ������ʢ��10gϡ������ձ��У����е�̼��Ƹ�����ǡ����ȫ��Ӧ�������ɷֲ������ᷴӦ�����ձ������ʵ���������Ϊ11.34g�����㣺
��ʯ��ʯ��̼��Ƶ�������
������ϡ��������Ч�ɷֵ�����������
��1�����������ļ��ٶ�����̼�Ĵ�ʩ��
��2��ijУ��ѧС���ͬѧΪ̽��������й����ŷŶ�����̼�Ի�����Ӱ�죬��������ʵ�飮ʵ������У����ǽ�����װ��ͬʱ������ͬ�Ļ�����������ͬ��ʱ�䣨�������еĺ�ɫҺ�岻������еijɷַ�����Ӧ��

��ʵ������пɹ۲쵽��������ʲô��
�ڷ�������ʵ������IJ���ɵõ��Ľ�����ʲô��
��3��2007��9��1�գ����ҷ���ί����������ʮ�߸�������ȫ����ʽ���𡰽��ܼ���ȫ���ж�����Ϊ�����Դ��ȱ�ͻ�����Ⱦ���⣬�д��������������õ�����Դ��
��4��ȡ2gʯ��ʯ������ʢ��10gϡ������ձ��У����е�̼��Ƹ�����ǡ����ȫ��Ӧ�������ɷֲ������ᷴӦ�����ձ������ʵ���������Ϊ11.34g�����㣺
��ʯ��ʯ��̼��Ƶ�������
������ϡ��������Ч�ɷֵ�����������
���㣺������̼�Ի�����Ӱ��,������̼�Ļ�ѧ����,�й��������������ļ���,���ݻ�ѧ��Ӧ����ʽ�ļ���,��Դ�ۺ����ú�����Դ����
ר�⣺�������������뻯ѧ����ʽ���ϵļ���,��ѧ����Դ,̼�����뺬̼���������������;
��������1��������̼��ʹȫ���ů����Ҫ�������壬Ҫ���ٶ�����̼������ŷţ���Ҫ�Ӷ�����̼������ԭ��ͼ��ٴ����ж�����̼�ķ����Ƕȿ��ǣ�
��2�����ݶ�����̼�������ƿ���¶ȸ߷������
��3�������ԴΣ����������������Դ�����н�������⣬������һ�ֿ������ͣ�ֻҪ�𰸺������ɣ�
��4��ʯ��ʯ��������ϡ�����������ȥ���ձ���ʣ�����ʵ��������õ����������ɶ�����̼��������
�ɶ�����̼����������̼�����ϡ���ᷴӦ�Ļ�ѧ����ʽ���Լ����ʯ��ʯ��̼��Ƶ�������ϡ���������ʵ�����������
��2�����ݶ�����̼�������ƿ���¶ȸ߷������
��3�������ԴΣ����������������Դ�����н�������⣬������һ�ֿ������ͣ�ֻҪ�𰸺������ɣ�
��4��ʯ��ʯ��������ϡ�����������ȥ���ձ���ʣ�����ʵ��������õ����������ɶ�����̼��������
�ɶ�����̼����������̼�����ϡ���ᷴӦ�Ļ�ѧ����ʽ���Լ����ʯ��ʯ��̼��Ƶ�������ϡ���������ʵ�����������
����⣺��1������������̼����Ҫԭ���ǻ�ʯȼ�ϵ�ȼ�գ����Ҫ����ʹ�û�ʯȼ�ϣ�ú��ʯ�͡���Ȼ������ʹ�ã�������������ȼ�ϣ�̫���ܡ����ܡ����ȵȣ������⣬ֲ���ܰѶ�����̼ת��Ϊ���������Ҫ����ֲ�����֣��Ͻ��ҿ��ķ�ɭ�ֵȣ�
�ʴ�Ϊ������ʹ�û�ʯȼ�ϣ�ú��ʯ�͡���Ȼ������������������ȼ�ϣ�̫���ܡ����ܡ����ȵȣ�������ֲ�����֣��Ͻ��ҿ��ķ�ɭ�ֵȣ�
��2��Bƿ�ж�����̼�������ƿ���¶ȸߣ�U��������ҺעҪ����Aƿ��˵��������̼��ʹȫ���ů����Ҫ�������壻
�ʴ�Ϊ��Bƿ��U��������ҺעҪ����Aƿ��˵��������̼��ʹȫ���ů����Ҫ�������壻
��3��Ŀǰ��Ҫ����������Դ�кܶ࣬�����ܵ�DZ�ܴܺ�̫���ܵȣ�
�ʴ�Ϊ�����ܡ�̫���ܣ�
��4�������ɶ�����̼������Ϊ��2g+10g-11.34g=0.66g��
��ʯ��ʯ��̼��Ƶ�����Ϊx��ϡ���������ʵ���������Ϊy��
CaCO3+2HCl=CaCl2+H2O+CO2��
100 73 44
x 10g��y 0.66g
=
��
=
��
x=1.5g��y=10.95%��
�𣺢�ʯ��ʯ��̼��Ƶ�����Ϊ1.5g��
��������Һ�����ʵ���������Ϊ10.95%��
�ʴ�Ϊ������ʹ�û�ʯȼ�ϣ�ú��ʯ�͡���Ȼ������������������ȼ�ϣ�̫���ܡ����ܡ����ȵȣ�������ֲ�����֣��Ͻ��ҿ��ķ�ɭ�ֵȣ�
��2��Bƿ�ж�����̼�������ƿ���¶ȸߣ�U��������ҺעҪ����Aƿ��˵��������̼��ʹȫ���ů����Ҫ�������壻
�ʴ�Ϊ��Bƿ��U��������ҺעҪ����Aƿ��˵��������̼��ʹȫ���ů����Ҫ�������壻
��3��Ŀǰ��Ҫ����������Դ�кܶ࣬�����ܵ�DZ�ܴܺ�̫���ܵȣ�
�ʴ�Ϊ�����ܡ�̫���ܣ�
��4�������ɶ�����̼������Ϊ��2g+10g-11.34g=0.66g��
��ʯ��ʯ��̼��Ƶ�����Ϊx��ϡ���������ʵ���������Ϊy��
CaCO3+2HCl=CaCl2+H2O+CO2��
100 73 44
x 10g��y 0.66g
| 100 |
| x |
| 44 |
| 0.66g |
| 73 |
| 10g��y |
| 44 |
| 0.66g |
x=1.5g��y=10.95%��
�𣺢�ʯ��ʯ��̼��Ƶ�����Ϊ1.5g��
��������Һ�����ʵ���������Ϊ10.95%��
�����������漰����Դ�����á�������̼�Ի�����Ӱ���Լ�����Դ�����úͿ�����һ����Ϣ�������ͣ�

��ϰ��ϵ�д�
�����Ŀ
 ijУ�о���ѧϰС������ͼװ�ý���þ���ڿ�����ȼ�յ�ʵ�飬ȼ�ա���ȴ���ֹˮ�У����뼯��ƿ�е�ˮ�����Լռ����ƿ�ݻ���70%��
ijУ�о���ѧϰС������ͼװ�ý���þ���ڿ�����ȼ�յ�ʵ�飬ȼ�ա���ȴ���ֹˮ�У����뼯��ƿ�е�ˮ�����Լռ����ƿ�ݻ���70%��

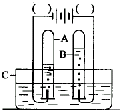 ��ͼ�ǵ��ˮ��ʾ��ͼ��
��ͼ�ǵ��ˮ��ʾ��ͼ��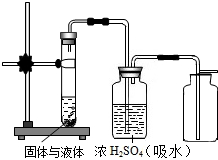 ������ͼװ�ûش�ʵ���ҿ��ø�װ����ȡ�����������
������ͼװ�ûش�ʵ���ҿ��ø�װ����ȡ�����������