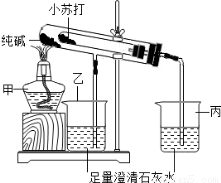
��Ŀ����
��7�֣�ijͬѧ��������ʵ�飺

ʵ�����ݼ�����ʵ���������±���
| ��һ�� | �ڶ��� |
������ͭ��������g�� | m | m |
��ϡ�����������g�� | 50 | 100 |
������������Һ��������g�� | 100 | 100 |
B����Ҫ���� | ����ɫ���� | �� |
����һ������ÿһ����ǡ����ȫ��Ӧ����������������Һ�����ʵ���������Ϊ8��55%����ش��������⣺
1��д����һ����������Һ��ɫ�ı�Ļ�ѧ��Ӧ����ʽ ��
2���ڶ���ʵ��B�е���Ҫ������ ��
3�������м�������ͭm����ֵΪ ��
4�����ڶ��η�Ӧ�����ɹ���������X���ı���ʽ ��
5�������ڶ��η�Ӧ�����Һ����32��35��ˮ�������ò�������Һ�����ʵ���������Ϊ ;
6������98%��Ũ���������������������ᣬ����Ҫ��ˮ������Ϊ ��
(1) CuO + H2SO4 = CuSO4 + H2O Ba(OH)2 +CuSO4 =Cu(OH)2�� + BaSO4��
(2)������ɫ����(��ɫ��Һ��dz) (3) 4
(4) 171��233=8��55g/X ��5��5% ��6��135g
��������
���������(1) ��һ��������������ͭ�м���ϡ���ᣬ������Ӧ��������ͭ��ɫ��Һ����ѧ��Ӧ����ʽΪ ��CuO + H2SO4 = CuSO4 + H2O�����ɵ�����ͭ��Һ����������������Һ��Ӧ����ѧ��Ӧ����ʽΪ ��Ba(OH)2 +CuSO4 =Cu(OH)2�� + BaSO4��
(2) ���ڵ�һ������ÿһ����ǡ����ȫ��Ӧ�����Եڶ���ʵ����� 100gϡ���ᣬ˵�����������Եڶ���ʵ��B�е���Ҫ������������ɫ����(��ɫ��Һ��dz)
(3)���ݻ�ѧ��Ӧ��CuO + H2SO4 = CuSO4 + H2O��Ba(OH)2 +CuSO4 =Cu(OH)2�� + BaSO4�������Կ���CuO��Ba(OH)2��������ϵ80��171������������������=100g��8��55%=8��55g�����Բ������CuO������m=4g
��4���ڶ��η�Ӧ�����ķ���ʽΪ��Ba(OH)2+H2SO4==BaSO4��+2H2O����Ba(OH)2��BaSO4��������ϵΪ171:233����Ba(OH)2������Ϊ100g��8��55%=8��55g�����Կ���BaSO4������Ϊx���ʿ���ʽΪ��171��233=8��55g/X
��5���ڶ��η�Ӧ�����Һ�Ǽ�����100gϡ���ᷴӦ�õ�����Һ �����ݵ�һ�η�Ӧ��������ϵ��CuO + H2SO4 = CuSO4 + H2O���ɼ��������CuSO4������Ϊ 8g������������ʽ��Ba(OH)2+H2SO4==BaSO4��+2H2O�������BaSO4������=11��65g������Һ����=4g+100g+100g-11��65g=192��35g,���������ڶ��η�Ӧ�����Һ����32��35��ˮ����ʱ��Һ��������Ϊ=192��35-32��35g=160g�������ò�������Һ�����ʵ���������=8g/160g��100%=5%
��6�����ݷ�ӦCuO + H2SO4 = CuSO4 + H2O��CuO��H2SO4��������ϵΪ80:98��CuO������m=4g������H2SO4������=4��9g������H2SO4����������=4��9g/50g��100%=9��8%�������ĵ�ϡ����������Ϊ150g�����Ը���ϡ��ǰ��������������䣬����98%��Ũ��������Ϊ x������ʽΪ ��98%��x=150g��9��8%,����x=15g,��ˮ������=150g-15g=135g
���㣺��ѧ��Ӧ����ʽ����д�����ݻ�ѧ��Ӧ����ʽ���㣬��Һ��ϡ��