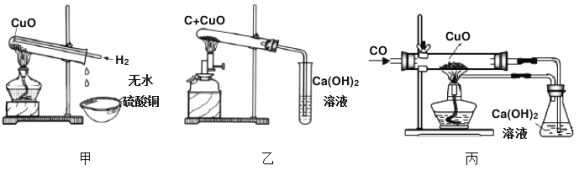
��Ŀ����
����Ŀ��̼Ԫ��������������ʵĻ���Ԫ�ء�
��1�����к�̼Ԫ�ص������У������л������ ___������ĸ��ţ���
A��̼��� B���Ҵ���C2H5OH�� C��������̼
��2����ʯȼ����Ҫ����ú�� ����Ȼ�������Ƕ�����̼Ԫ�أ�������Ȼ������Ҫ�ɷ��� ��д��ѧʽ����
��3���ܶ���Ȼ��ʯ�к���̼Ԫ�أ����̿����Ҫ�ɷ���̼���̣�MnCO3����������Ԫ�صĻ��ϼ� ��
��4���� 440����ѹ�����£��������������̼��Ӧ�����ɽ��ʯ��C����̼���ƣ��÷�Ӧ�Ļ�ѧ����ʽΪ ��
��5���������ʯ�ͼ������Կɻָ�ԭ����֪����Ĺ�����ϣ�����ͼ��ʾ������̼Ԫ����ɣ����ж�ṹ�����Ժá�����ʯ���к�ǿ����������������ˮ�����������ʯ�ͼ������Կɻָ�ԭ״�����й���̼�����˵����ȷ���� ������ĸ��ţ���

A������������
B�����ظ�ʹ��
C���ɴ�������ʯ��й©
���𰸡���1��B
��2��ʯ�� CH4
��3��+2
��4��4Na+3CO2![]() 3C+2Na2CO3
3C+2Na2CO3
��5��ABC
����������1���л�����ָ����̼Ԫ�صĻ������������CO��CO2��H2CO3��̼���ε�
��2����ȼ�ϵĿ���
��3����Mn�Ļ��ϼ�ΪX,���ݻ������л��ϼ�Ϊ0��ԭ�����X+(-2)=0 X= +2
��4�����������Ӧ��������Ӧ��������
��5����̼������ʯ���к�ǿ�������������ʾ��������ԣ���̼�������������ʯ�ͼ������Կɻָ�ԭ״���ʿ��ظ�ʹ�ã���Ȼ������ʯ�ͣ��ʿɴ�������ʯ��й©

 һ����ʦ�����Ծ�ϵ�д�
һ����ʦ�����Ծ�ϵ�д�