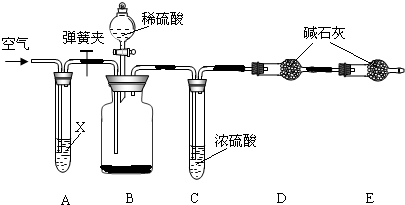
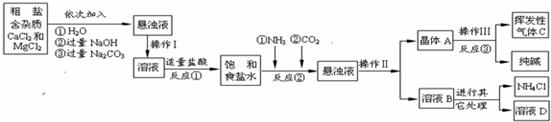
��Ŀ����
�������Ƽ���ƵõĴ�����ͨ�������������Ȼ��ƣ�ijͬѧ��ⶨ�����Ȼ������ʵĴ�����Ʒ�����ʵ�������������ʦ���������Լ���ϡ���ᡢ̼�����Һ���Ȼ�����Һ����ͬѧ�������£�ȡ��Ʒ5gȫ���ܽ���20gˮ�У���������Һ�м���29gij��ѡ�Լ���ǡ����ȫ��Ӧ�����˺�Ƶ���Һ����Ϊ50g���Իش�
��1��������Ӧ�Ļ�ѧ����ʽ��______��
��2�������Ʒ�д���������x���ı���ʽΪ______��
��3����Ʒ�������Ȼ��Ƶ���������Ϊ______��
��4��������Һ�����ʵ���������______��
��1��������Ӧ�Ļ�ѧ����ʽ��______��
��2�������Ʒ�д���������x���ı���ʽΪ______��
��3����Ʒ�������Ȼ��Ƶ���������Ϊ______��
��4��������Һ�����ʵ���������______��
��1���Ȼ�����̼���Ʒ�Ӧ�Ļ�ѧ����ʽΪ��Na2CO3+CaCl2=CaCO3��+2NaCl��
��2������뷴Ӧ��̼���Ƶ�����Ϊx�����ɵ��Ȼ��Ƶ�����Ϊy��
Na2CO3+CaCl2=CaCO3��+2NaCl
106 100 117
x 4g y
��106��100=x��4g��
100��117=4g��y��
��֮�ã�x=4.24g��y=4.68g��
��3����Ʒ�������Ȼ��Ƶ���������Ϊ��
��100%=15.2%��
��4��������Һ�����ʵ�����Ϊ��4.68g+5g-4.24g=5.44g��
������Һ�����ʵ���������Ϊ��
��100%=10.88%��
�ʴ�Ϊ����1��Na2CO3+CaCl2=CaCO3��+2NaCl����2��106��100=x��4g����3��15.2%����4��10.88%��
��2������뷴Ӧ��̼���Ƶ�����Ϊx�����ɵ��Ȼ��Ƶ�����Ϊy��
Na2CO3+CaCl2=CaCO3��+2NaCl
106 100 117
x 4g y
��106��100=x��4g��
100��117=4g��y��
��֮�ã�x=4.24g��y=4.68g��
��3����Ʒ�������Ȼ��Ƶ���������Ϊ��
| 5g-4.24g |
| 5g |
��4��������Һ�����ʵ�����Ϊ��4.68g+5g-4.24g=5.44g��
������Һ�����ʵ���������Ϊ��
| 5.44g |
| 50g |
�ʴ�Ϊ����1��Na2CO3+CaCl2=CaCO3��+2NaCl����2��106��100=x��4g����3��15.2%����4��10.88%��

��ϰ��ϵ�д�
 ��������ϵ�д�
��������ϵ�д� ����˼ά����ѵ����ʱ��ѧ��ϵ�д�
����˼ά����ѵ����ʱ��ѧ��ϵ�д�
�����Ŀ