��Ŀ����
������ʹ�����Ľ������ϡ�
(1)�����ֶ������Ͻ����к�̼���ϸߵ���_______________��
(2)���Ǵ���ʹ�õ������Ͻ�����Ǵ�����������Ϊ���ĺϽ����
�����������ܣ�����ֱȴ���Ӳ�� �����С������
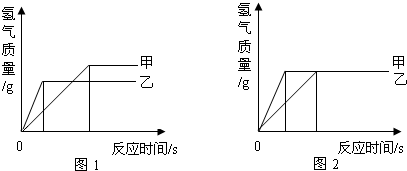
(3) ��ͼ��ʾ��Һ̬������̼���������ش��������⡣
��ͼ�����������������л����ϵ���_____________��ֻдһ�֣���
�ڼ�ѹ��С��ƿ��Һ̬������̼������������ԭ����
__________________________________��_____________________________________��
(4)����������������Ҫԭ�ϣ�д����CO�ͳ����������Ļ�ѧ����ʽ ��

��1������ ��2���� ��3�������ϡ����� ���������Ƚ���ʹ�����¶ȴﲻ���Ż��
CO2�����ڿ�ȼ��������O2 ��4��3CO + Fe2O3 = 2Fe + 3CO2

��ϰ��ϵ�д�
�����Ŀ
 ������ʹ�����Ľ������ϣ�
������ʹ�����Ľ������ϣ�
