��Ŀ����
 17���Ķ����ϣ��ش����⣺
17���Ķ����ϣ��ش����⣺���ϣ�l875�꣬������ѧ�Ҳ��߲����ʹ����������ֵ���Ԫ���أ�Ԫ�ط���ΪGa����������������ɫ�������ܶ�4.7g?cm-3�������ˮ���ҷ�Ӧ�����������������أ��ص�ԭ�ӽṹʾ��ͼ���ң��������ϼ�Ϊ+3��
��1����Ԫ�ص�������Ϊ
31
����ԭ������������Ϊ3
����Ԫ��λ��Ԫ�����ڱ��ĵ���
���ڣ���2��������������ϣ��ܽ�����ص��й�֪ʶ��
�������ʣ�
����ɫ�������ܶ�Ϊ5.94g?cm-3
����ѧ���ʣ����û�ѧ����ʽ��ʾ����
2Ga+6H2O=Ga��OH��3+3H2��
����3���������ؾ��м�����ʣ�������������ϡ���ᷴӦ�����εĻ�ѧʽΪ
Ga2��SO4��3
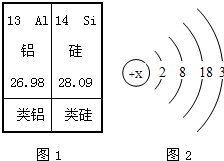
����������1�����ص�ԭ�ӽṹʾ��ͼ��֪������������=��������������֪��Ԫ�ص������������������������ݵ��Ӳ�������֪����������
��2������������ָ���ʲ���Ҫ������ѧ�仯�ͱ��ֳ��������ʣ���ѧ������ָ�����ڻ�ѧ�仯�б��ֳ��������ʣ�����ѧ�仯�ı��������DZ仯�������������ɣ������⣬��֪���������ʺͻ�ѧ���ʣ�
��3����֪ʶǨ�����Ƶķ�������д����������ϡ���ᷴӦ�����εĻ�ѧʽ����Ϊ��Ԫ��ԭ������������Ϊ3�����仯�ϼ�Ϊ+3�ۣ���������������������������������������ᷴӦ�Ļ�ѧ����ʽΪ��3H2SO4+2Al��OH��3=Al2��SO4��3+6H2O���ʿ���д����������ϡ���ᷴӦ�Ļ�ѧ����ʽ��
��2������������ָ���ʲ���Ҫ������ѧ�仯�ͱ��ֳ��������ʣ���ѧ������ָ�����ڻ�ѧ�仯�б��ֳ��������ʣ�����ѧ�仯�ı��������DZ仯�������������ɣ������⣬��֪���������ʺͻ�ѧ���ʣ�
��3����֪ʶǨ�����Ƶķ�������д����������ϡ���ᷴӦ�����εĻ�ѧʽ����Ϊ��Ԫ��ԭ������������Ϊ3�����仯�ϼ�Ϊ+3�ۣ���������������������������������������ᷴӦ�Ļ�ѧ����ʽΪ��3H2SO4+2Al��OH��3=Al2��SO4��3+6H2O���ʿ���д����������ϡ���ᷴӦ�Ļ�ѧ����ʽ��
����⣺��1�����ص�ԭ�ӽṹʾ��ͼ��֪����Ԫ�ص�������Ϊ31����ԭ������������Ϊ3������Ӳ���Ϊ4������Ԫ��λ��Ԫ�����ڱ��ĵ������ڣ�
��2��������������ϣ��ܽ�����ص��й�֪ʶ��
�������ʣ�����ɫ�������ܶ�Ϊ5.94g?cm-3��
��ѧ���ʣ����û�ѧ����ʽ��ʾ�����������ˮ���ҷ�Ӧ�����������������ء�����Ϊ��Ԫ��ԭ������������Ϊ3�����仯�ϼ�Ϊ+3�ۣ���2Ga+6H2O=Ga��OH��3+3H2����
��3����֪ʶǨ�����Ƶķ�������д����������ϡ���ᷴӦ�����εĻ�ѧʽ����Ϊ��Ԫ��ԭ������������Ϊ3�����仯�ϼ�Ϊ+3�ۣ���������������������������������������ᷴӦ��ѧ����ʽΪ��3H2SO4+2Al��OH��3=Al2��SO4��3+6H2O���ʿ���д����������ϡ���ᷴӦ�Ļ�ѧ����ʽ3H2SO4+2Ga��OH��3=Ga2��SO4��3+6H2O�������������ؾ��м�����ʣ�������������ϡ���ᷴӦ�����εĻ�ѧʽΪ Ga2��SO4��3 ��
�ʴ�Ϊ����1��31��3���ģ�
��2������ɫ�������ܶ�Ϊ5.94g?cm-3��2Ga+6H2O=Ga��OH��3+3H2����
��3��Ga2��SO4��3
��2��������������ϣ��ܽ�����ص��й�֪ʶ��
�������ʣ�����ɫ�������ܶ�Ϊ5.94g?cm-3��
��ѧ���ʣ����û�ѧ����ʽ��ʾ�����������ˮ���ҷ�Ӧ�����������������ء�����Ϊ��Ԫ��ԭ������������Ϊ3�����仯�ϼ�Ϊ+3�ۣ���2Ga+6H2O=Ga��OH��3+3H2����
��3����֪ʶǨ�����Ƶķ�������д����������ϡ���ᷴӦ�����εĻ�ѧʽ����Ϊ��Ԫ��ԭ������������Ϊ3�����仯�ϼ�Ϊ+3�ۣ���������������������������������������ᷴӦ��ѧ����ʽΪ��3H2SO4+2Al��OH��3=Al2��SO4��3+6H2O���ʿ���д����������ϡ���ᷴӦ�Ļ�ѧ����ʽ3H2SO4+2Ga��OH��3=Ga2��SO4��3+6H2O�������������ؾ��м�����ʣ�������������ϡ���ᷴӦ�����εĻ�ѧʽΪ Ga2��SO4��3 ��
�ʴ�Ϊ����1��31��3���ģ�
��2������ɫ�������ܶ�Ϊ5.94g?cm-3��2Ga+6H2O=Ga��OH��3+3H2����
��3��Ga2��SO4��3
�������˽���ӡ�ԭ�ӡ����ӡ�Ԫ��������֮��Ĺ�ϵ���˽ⳣ��Ԫ�غ�ԭ���ŵĻ��ϼۣ���Χ�������ʺͻ�ѧ���ʵIJ����Ӧ�ã�

��ϰ��ϵ�д�
 �������Ͽ��㱾ϵ�д�
�������Ͽ��㱾ϵ�д�
�����Ŀ
 �Ķ����ϣ��ش����⣺
�Ķ����ϣ��ش����⣺