��Ŀ����
 �����벻����ѧ�������벻��ˮ��
�����벻����ѧ�������벻��ˮ��
���û�ѧʽ��գ�
����θҺ�к��е�����________��
�γ��������Ҫ������________��
�к��������Ե�������________��
��ҽ���ϳ���75%�ľƾ���C2H5OH����Һ�����������ƾ�Ħ��������________������̼���⡢��ԭ�����ʵ���֮��Ϊ________��1.5molC2H5OH������Լ����
________��̼ԭ�ӣ�
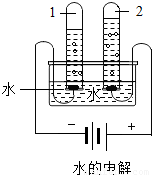
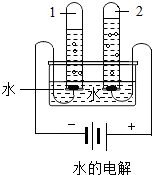
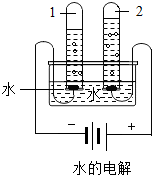
����ͼˮ���ʵ���У��Թ�2�еõ���������________������ˮ������ͨ��������������________�����þ�ˮ����װ�������Ե�������________��д�������ƣ���
HCl SO2 Ca��OH��2 46g/mol 2��6��1 1.806��1024 O2�������� ɱ������ ����̿
�����������ȸ�������ȷ�����ʵĻ�ѧ���ƣ�Ȼ�������д��ѧʽ�ķ����Ͳ���д�����ʵĻ�ѧʽ���ɣ�
��Ħ��������ָ��λ���ʵ��������������е�������Ħ�������ĵ�λΪg/mol������ֵ�ϵ��ڸ����ʵ����ԭ����������Է����������ݴ˷������㼴�ɣ�1mol�κ������к���6.02��1023�����ӣ�
�۸��ݵ��ˮ��ʵ����������ˮ����ˮ�ķ����Ƚ��з�����ɣ�
��𣺢�����θҺ�к��е�����Ԫ�أ��仯ѧʽΪ��HCl��
�����������γ��������Ҫ���壬�仯ѧʽΪ��SO2��
�������ƾ��м��ԣ����ڼ�����ڸ��������������仯ѧʽΪ��Ca��OH��2��
��Ħ��������ָ��λ���ʵ��������������е��������ƾ�����Է���������46���ʾƾ���Ħ��������46g/mol��
һ���ƾ���������2��̼ԭ�ӡ�6����ԭ�Ӻ�1����ԭ�ӣ�������̼���⡢��ԭ�����ʵ���֮��Ϊ2��6��1��
1mol�κ������к���6.02��1023�����ӣ�һ���ƾ������к���2��̼ԭ�ӣ�1.5molC2H5OH������Լ����̼ԭ�ӵĸ���Ϊ1.5mol��2��6.02��1023��=1.806��1024��
�۵��ˮʱ�����Դ�����������Թ��ڲ�������������٣������������Դ�����������Թ��ڵ���������࣬���������Թ�2���Դ�������������õ���������������
����ˮ������ͨ��������������ɱ��������
����̿���������ԣ���������ζ��ɫ�أ������ڼ��þ�ˮ���У�
�ʴ�Ϊ����HCl��SO2��Ca��OH��2����46g/mol��2��6��1��1.806��1024����O2����������ɱ������������̿��
�����������ѶȲ������ճ������ʵ����ʡ���;����ɼ���ѧʽ����д���йػ�ѧʽ�ļ��������ȷ�����Ĺؼ����ڣ�
�����������ȸ�������ȷ�����ʵĻ�ѧ���ƣ�Ȼ�������д��ѧʽ�ķ����Ͳ���д�����ʵĻ�ѧʽ���ɣ�
��Ħ��������ָ��λ���ʵ��������������е�������Ħ�������ĵ�λΪg/mol������ֵ�ϵ��ڸ����ʵ����ԭ����������Է����������ݴ˷������㼴�ɣ�1mol�κ������к���6.02��1023�����ӣ�
�۸��ݵ��ˮ��ʵ����������ˮ����ˮ�ķ����Ƚ��з�����ɣ�
��𣺢�����θҺ�к��е�����Ԫ�أ��仯ѧʽΪ��HCl��
�����������γ��������Ҫ���壬�仯ѧʽΪ��SO2��
�������ƾ��м��ԣ����ڼ�����ڸ��������������仯ѧʽΪ��Ca��OH��2��
��Ħ��������ָ��λ���ʵ��������������е��������ƾ�����Է���������46���ʾƾ���Ħ��������46g/mol��
һ���ƾ���������2��̼ԭ�ӡ�6����ԭ�Ӻ�1����ԭ�ӣ�������̼���⡢��ԭ�����ʵ���֮��Ϊ2��6��1��
1mol�κ������к���6.02��1023�����ӣ�һ���ƾ������к���2��̼ԭ�ӣ�1.5molC2H5OH������Լ����̼ԭ�ӵĸ���Ϊ1.5mol��2��6.02��1023��=1.806��1024��
�۵��ˮʱ�����Դ�����������Թ��ڲ�������������٣������������Դ�����������Թ��ڵ���������࣬���������Թ�2���Դ�������������õ���������������
����ˮ������ͨ��������������ɱ��������
����̿���������ԣ���������ζ��ɫ�أ������ڼ��þ�ˮ���У�
�ʴ�Ϊ����HCl��SO2��Ca��OH��2����46g/mol��2��6��1��1.806��1024����O2����������ɱ������������̿��
�����������ѶȲ������ճ������ʵ����ʡ���;����ɼ���ѧʽ����д���йػ�ѧʽ�ļ��������ȷ�����Ĺؼ����ڣ�

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ
 ��2013?�ɽ�����ģ�������벻����ѧ�������벻��ˮ��
��2013?�ɽ�����ģ�������벻����ѧ�������벻��ˮ�� ��
��  �������ƾ�Ħ�������� (4) ������̼���⡢��ԭ�����ʵ���֮��Ϊ (5) ��1.5molC2H5OH������Լ���� (6) ��̼ԭ�ӡ�
�������ƾ�Ħ�������� (4) ������̼���⡢��ԭ�����ʵ���֮��Ϊ (5) ��1.5molC2H5OH������Լ���� (6) ��̼ԭ�ӡ�