��Ŀ����
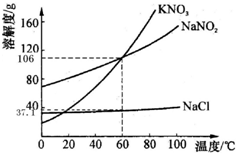 ��2010?������һģ����ͼΪKNO3��NaNO2���������ƣ���NaCl���ܽ�����ߣ���������ش��������⣺
��2010?������һģ����ͼΪKNO3��NaNO2���������ƣ���NaCl���ܽ�����ߣ���������ش��������⣺��1��60��ʱ��KNO3�ı�����Һ�����ʵ���������
����
����
������ڡ��������ڡ���С�ڡ���NaNO2������Һ�����ʵ�������������2��60��ʱ��ȡNaNO2��NaCl���ֹ����50g���ֱ���뵽100gˮ�г�ֽ��裬û����ȫ�ܽ�Ĺ�����
NaCl
NaCl
��δ�ܽ�Ĺ�������Ϊ12.9
12.9
g��������ȫ�ܽ����Һ����������
������
������͡������͡�����Һ������Һ��������������Ϊ33.3%
33.3%
������1λС�����������������ܽ�����ߵ������ϱ�����Һ�Ͳ�������Һ�Ķ����������⣮
����⣺��1�������ܽ�����ߵ��������֪������60��ʱ����غ��������Ƶ��ܽ����ȣ����Ը����ܽ�ȶ�����Եó�����60��ʱ������غ��������Ƶı�����Һ�����ʵ�����������ȣ�
��2�������ܽ�����ߵ��������֪������60��ʱ���Ȼ��Ƶ��ܽ��Ϊ37.1g���ʴ�ʱ��100gˮ�м���50g�Ȼ���ֻ���ܽ�37.1g�������ܽ���Ȼ��Ƶ�����Ϊ50g-37.1g=12.9g������60��ʱ���������Ƶ��ܽ��Ϊ106g�����Դ�ʱ�õ�����ҺΪ��������Һ�������ʵ���������Ϊ��
��100%=33.3%��
�ʴ�Ϊ����1�����ڣ�
��2��NaCl��12.9g�������ͣ�33.3%��
��2�������ܽ�����ߵ��������֪������60��ʱ���Ȼ��Ƶ��ܽ��Ϊ37.1g���ʴ�ʱ��100gˮ�м���50g�Ȼ���ֻ���ܽ�37.1g�������ܽ���Ȼ��Ƶ�����Ϊ50g-37.1g=12.9g������60��ʱ���������Ƶ��ܽ��Ϊ106g�����Դ�ʱ�õ�����ҺΪ��������Һ�������ʵ���������Ϊ��
| 50g |
| 100g+50g |
�ʴ�Ϊ����1�����ڣ�
��2��NaCl��12.9g�������ͣ�33.3%��
��������Ҫ�����˶Թ����ܽ�ȵĸ������������ܽ�����ߵ����壬�Դ�����ѧ������������������ѧ���������⡢��������������

��ϰ��ϵ�д�
 â���̸������Ծ�ϵ�д�
â���̸������Ծ�ϵ�д�
�����Ŀ