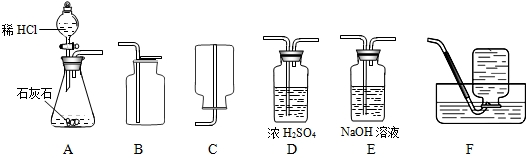
��Ŀ����
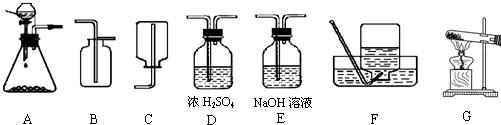
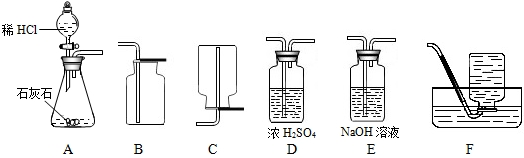
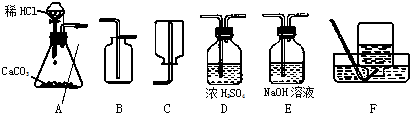
4��Ϊ���о�CO2�����ʣ���Ҫ��ȡ���ռ������CO2���壬��������ʦ�ṩ��һЩʵ��װ�ã���ʾŨ���������ˮ���ܰ������е�ˮ�������ն���ȥ��NaOH��Һ�ܺͶ�����̼��Ӧ�ȣ���

��1����ȡ���ռ������CO2���壬�ɲ��õ�װ�������
��2��ʵ������ȡCO2�Ļ�ѧ����ʽΪ
��3����������ʵ�����������ռ���������ܵ�ԭ����

��1����ȡ���ռ������CO2���壬�ɲ��õ�װ�������
A��D��B
������ĸ������2��ʵ������ȡCO2�Ļ�ѧ����ʽΪ
CaCO3+2HCl=CaCl2+CO2��+H2O
����3����������ʵ�����������ռ���������ܵ�ԭ����
ҩƷ������������Բ��õ�
����������1���ٶ�����̼����ȡ������+Һ�巴Ӧ��������ȣ�ѡ��Aװ��Ϊ��ȡװ�ã��ڸ����CO2���壺��Dװ�ã�����Ũ�������ˮ���ܰ������е�ˮ�������ն���ȥ�������ռ�������̼����ֻ���������ſ�������������Ϊ���ܶȱȿ�����ѡ��Bװ�ã�
��2����ȡ��д��ѧ����ʽ��CaCO3+2HCl=CaCl2+CO2��+H2O
��3����С�⣬Ϊ��������Ŀ�����ܵ�ԭ���ж��ִ𰸣�
��2����ȡ��д��ѧ����ʽ��CaCO3+2HCl=CaCl2+CO2��+H2O
��3����С�⣬Ϊ��������Ŀ�����ܵ�ԭ���ж��ִ𰸣�
����⣺��1��Aװ��Ϊ��ȡװ�ã�����©��Ϊ��Һ©��������©�����ŵ��ǿ��Կ���Һ��ĵμ��ٶȣ����������̼����Ӧ����Dװ�ã�����Ũ�������ˮ�ԣ�����������������Һ����ΪNaOH��Һ�ܺͶ�����̼��Ӧ���ռ�������̼����ֻ���������ſ�������������Ϊ������̼�����ܶȱȿ�������������ˮ��
��2����ȡ��д��ѧ����ʽ��
��3��ʵ�����������ռ���������ܵ�ԭ���ǣ����ִ𰸣�
�ʴ�Ϊ����1��A��D��B ��2�� CaCO3+2HCl=CaCl2+CO2��+H2O ��3��ҩƷ������������Բ��õ�
��2����ȡ��д��ѧ����ʽ��
��3��ʵ�����������ռ���������ܵ�ԭ���ǣ����ִ𰸣�
�ʴ�Ϊ����1��A��D��B ��2�� CaCO3+2HCl=CaCl2+CO2��+H2O ��3��ҩƷ������������Բ��õ�
����������Ϊʵ������ȡ������̼��һ���ۺ��⣬���������̼��ʵ�����Ʒ�ԭ������ȡװ�á��ռ�װ�õ�ѡ���Լ�������̼��ijЩ���ʣ�NaOH��Һ�ܺͶ�����̼��Ӧ��

��ϰ��ϵ�д�
�����Ŀ