��Ŀ����
�����������ܽ⵰���ʺ���֬����Ƥ����ֽ�š�֯�����ǿ��ʴ�ԣ���ʹ��ʱҪ��ֹ�����۾���մ��Ƥ���ϣ�����ʵ������������˽��������Ƶ��������ʣ�
(1)
��������ͷ��(��Ҫ�ɷ��ǵ�����)����Ũ����������Һ�м��ȣ�(2)
��һ�鲼�Ϸ���Ũ����������Һ�У����裮(3)
������������Һ�еμ�1��2��ʳ�ͣ�������������ѧ����֪ʶ���ṩ��������Ԥ��һ��ʵ���лῴ��������
�𰸣���
������
��ʾ��
������
|
(1) ͷ�����ܽ�(2) �������ã�����һ���͵�����(3) ʳ������Ҳ�ܽ��� |
��ʾ��
|
�����Ŀ�Ĵ𰸼����Ѿ����ṩ���������ˣ�ֻҪ�л����Ķ������������ܽ�����⣮����������Һ���ܽ⵰���ʺ���֬����ʵ�� (1)��(3)������϶��ǣ�ͷ�����ܽ⣻ʳ������Ҳ�ܽ��ˣ�����Ϊ����������Һ��Ƥ����ֽ�š�֯�����ǿ��ʴ�ԣ�����ʵ��(2)������Ӧ���ǣ��������ã�����һ���͵����ˣ� |

��ϰ��ϵ�д�
�����Ŀ
�����д����л�ѧ����ѧ�ٽ����������ķ�չ���ٽ���������Ȼ��г�ദ��

��1������Ӫ������������
����ͼ1��ʾ��ʳ����գ�����дһ�֣�
���������ʵ���________���������۵���________������ά����C����________��
��2����ѧ�������ǵ�����������
��������Ʒ�У�________�����л��ϳɲ����Ƴɵģ����ţ���
A�������·����� B��������ˢ��������C��ľ����ߡ������� D����������
��һ�����͵�ʳƷ���ʼ�--��ĭ���ۣ������ӳ�ʳƷ�ı����ڣ����۶�ʳƷ���б������õ�ԭ����________�����ţ���
A�����ǻ�ѧ���ʺ��ȶ��Ľ���
B����������ʱ����ˮ�֣���ʹʳƷ���ָ���
C����������ʱ������������ֹʳƷ������������
��3�����������ռ������Ա��Ϊ����������Ⱦ����ͼ2�����������־����������������ࣺ
ֽ�ʰ�װ�С�����ƿ������������________�����ţ���ͬ�����ϵ�ء���ӫ��ƹܡ�����ҩƷ������________��������������Ҷ������________��
4 ijƷ�ƹܵ���ͨ���IJ�Ʒ���������ʾ��
| ��Ʒ���� | XXX�ܵ���ͨ�� |
| ��Ʒ�ɷ� | �������ơ���̼���ơ�ƫ�����ơ�������Լ��� |
| ��Ʒ���� | �������������ӿɿ����ܽ���֬��ë�����л��� |
| ���÷�Χ | ������������PVC���ʵ���ˮ�ܵ������������ܡ����� |
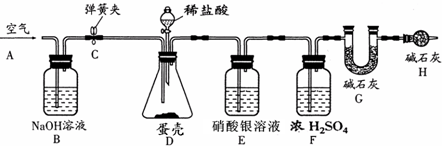
�ڹܵ���ͨ����������������ͨ����Ϊ������Һ�ḯʴ���ܣ���Ӧ�Ļ�ѧ����ʽΪ2Al+2NaOH+2X=2NaAlO2+3H2����X�Ļ�ѧʽΪ________��
�����д����л�ѧ����ѧ�ٽ����������ķ�չ���ٽ���������Ȼ��г�ദ��

��1������Ӫ������������
����ͼ1��ʾ��ʳ����գ�����дһ�֣�
���������ʵ��� ���������۵��� ������ά����C���� ��
��2����ѧ�������ǵ�����������
��������Ʒ�У� �����л��ϳɲ����Ƴɵģ����ţ���
A�������·� B��������ˢ C��ľ����� D����������
��һ�����͵�ʳƷ���ʼ�--��ĭ���ۣ������ӳ�ʳƷ�ı����ڣ����۶�ʳƷ���б������õ�ԭ���� �����ţ���
A�����ǻ�ѧ���ʺ��ȶ��Ľ���
B����������ʱ����ˮ�֣���ʹʳƷ���ָ���
C����������ʱ������������ֹʳƷ������������
��3�����������ռ������Ա��Ϊ����������Ⱦ����ͼ2�����������־����������������ࣺ
ֽ�ʰ�װ�С�����ƿ������������ �����ţ���ͬ�����ϵ�ء���ӫ��ƹܡ�����ҩƷ������ ��������������Ҷ������ ��
4 ijƷ�ƹܵ���ͨ���IJ�Ʒ���������ʾ��
��ʹ�ùܵ���ͨ������ˮ����Һ��pH �����7����=7����7������
�ڹܵ���ͨ����������������ͨ����Ϊ������Һ�ḯʴ���ܣ���Ӧ�Ļ�ѧ����ʽΪ2Al+2NaOH+2X=2NaAlO2+3H2����X�Ļ�ѧʽΪ ��

��1������Ӫ������������
����ͼ1��ʾ��ʳ����գ�����дһ�֣�
���������ʵ��� ���������۵��� ������ά����C���� ��
��2����ѧ�������ǵ�����������
��������Ʒ�У� �����л��ϳɲ����Ƴɵģ����ţ���
A�������·� B��������ˢ C��ľ����� D����������
��һ�����͵�ʳƷ���ʼ�--��ĭ���ۣ������ӳ�ʳƷ�ı����ڣ����۶�ʳƷ���б������õ�ԭ���� �����ţ���
A�����ǻ�ѧ���ʺ��ȶ��Ľ���
B����������ʱ����ˮ�֣���ʹʳƷ���ָ���
C����������ʱ������������ֹʳƷ������������
��3�����������ռ������Ա��Ϊ����������Ⱦ����ͼ2�����������־����������������ࣺ
ֽ�ʰ�װ�С�����ƿ������������ �����ţ���ͬ�����ϵ�ء���ӫ��ƹܡ�����ҩƷ������ ��������������Ҷ������ ��
4 ijƷ�ƹܵ���ͨ���IJ�Ʒ���������ʾ��
| ��Ʒ���� | XXX�ܵ���ͨ�� |
| ��Ʒ�ɷ� | �������ơ���̼���ơ�ƫ�����ơ�������Լ��� |
| ��Ʒ���� | �������������ӿɿ����ܽ���֬��ë�����л��� |
| ���÷�Χ | ������������PVC���ʵ���ˮ�ܵ������������ܡ����� |
�ڹܵ���ͨ����������������ͨ����Ϊ������Һ�ḯʴ���ܣ���Ӧ�Ļ�ѧ����ʽΪ2Al+2NaOH+2X=2NaAlO2+3H2����X�Ļ�ѧʽΪ ��