��Ŀ����
��ѧ���������ߣ���������ѧ�Ļ�ѧ֪ʶ����������⣺
��1����ʯȼ�ϰ���ú��ʯ�ͺ���Ȼ������ʯȼ�ϵ��ص���______������ĸ��ţ���
A���������� B�����ȼ�� C����Դ���� D��������Դ
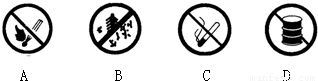
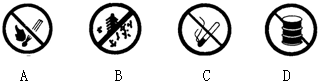
��2��������ͼ���У���������վ�������ú��䱸����______������ĸ��ţ���

��3�������Ҵ����Ϳɽ�ʡʯ����Դ����������β������Ⱦ���Ҵ��ڿ�����ȼ�յĻ�ѧ����ʽΪ______��

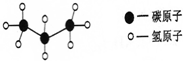
��4�����˻����2008��6��8���ڹ�����ɫ�д��ݣ����˻���ȼ����ҪΪ���飬������ӵĽṹģ����ͼ��ʾ�������Ļ�ѧʽΪ______��
��5�����������á������С����ܼ��š��Ĵ�ʩ֮һ��С���ĸ�������Ӱ������Ķ�ӰҺ���ں�AgNO3��Һ�������۷�Ӧ�ɻ��ս������������¸��ĵ�·���÷�Ӧ�Ļ�ѧ����ʽΪ______��
��1����ʯȼ�ϰ���ú��ʯ�ͺ���Ȼ������ʯȼ�ϵ��ص���______������ĸ��ţ���
A���������� B�����ȼ�� C����Դ���� D��������Դ
��2��������ͼ���У���������վ�������ú��䱸����______������ĸ��ţ���

��3�������Ҵ����Ϳɽ�ʡʯ����Դ����������β������Ⱦ���Ҵ��ڿ�����ȼ�յĻ�ѧ����ʽΪ______��

��4�����˻����2008��6��8���ڹ�����ɫ�д��ݣ����˻���ȼ����ҪΪ���飬������ӵĽṹģ����ͼ��ʾ�������Ļ�ѧʽΪ______��
��5�����������á������С����ܼ��š��Ĵ�ʩ֮һ��С���ĸ�������Ӱ������Ķ�ӰҺ���ں�AgNO3��Һ�������۷�Ӧ�ɻ��ս������������¸��ĵ�·���÷�Ӧ�Ļ�ѧ����ʽΪ______��
��1�����ݻ�ʯȼ��Ϊ����������Դ����ѡA��
��2������վ���д�����ȼ�ͣ���ȼ��������ᱻ��ȼ��
A����ʾ��ֹ�̻𣬹�Ӧ���ã�
B����ʾ��ֹ�ű��ڣ���Ӧ���ã�
C����ֹ���̣���Ӧ���ã�
D�����ͱ���������ȼ��ʲ������ã� ��ѡD��
��3��������д��ѧ����ʽ�IJ��裬�Ҵ��ڿ�����ȼ�յĻ�ѧ����ʽΪ��C2H5OH+3O2
2CO2+3H2O��
�ʴ�Ϊ��C2H5OH+3O2
2CO2+3H2O��
��4�����ݱ�����ӵĽṹģ�ͣ���֪����Ļ�ѧʽΪ��C3H8 ���ʴ�Ϊ��C3H8 ��
��5��������д��ѧ����ʽ�IJ��裬AgNO3��Һ�����۷�Ӧ�Ļ�ѧ����ʽΪ��Fe+2AgNO3=Fe��NO3��2+2Ag��
�ʴ�Ϊ��Fe+2AgNO3=Fe��NO3��2+2Ag��
��2������վ���д�����ȼ�ͣ���ȼ��������ᱻ��ȼ��
A����ʾ��ֹ�̻𣬹�Ӧ���ã�
B����ʾ��ֹ�ű��ڣ���Ӧ���ã�
C����ֹ���̣���Ӧ���ã�
D�����ͱ���������ȼ��ʲ������ã� ��ѡD��
��3��������д��ѧ����ʽ�IJ��裬�Ҵ��ڿ�����ȼ�յĻ�ѧ����ʽΪ��C2H5OH+3O2
| ||
�ʴ�Ϊ��C2H5OH+3O2
| ||
��4�����ݱ�����ӵĽṹģ�ͣ���֪����Ļ�ѧʽΪ��C3H8 ���ʴ�Ϊ��C3H8 ��
��5��������д��ѧ����ʽ�IJ��裬AgNO3��Һ�����۷�Ӧ�Ļ�ѧ����ʽΪ��Fe+2AgNO3=Fe��NO3��2+2Ag��
�ʴ�Ϊ��Fe+2AgNO3=Fe��NO3��2+2Ag��

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
 ��2012?��ɽ����ģ����ѧ�����������ߣ���������ѧ�Ļ�ѧ֪ʶ���ش��������������е����⣮
��2012?��ɽ����ģ����ѧ�����������ߣ���������ѧ�Ļ�ѧ֪ʶ���ش��������������е����⣮