��Ŀ����
 ��ͼ����ƿ��ʢ��98%�����ᣨ�ܶ�Ϊ1.84g/cm3������Һ©���Ļ�������ˮ������ƿ�У����Կ�����ƿ�г���
��ͼ����ƿ��ʢ��98%�����ᣨ�ܶ�Ϊ1.84g/cm3������Һ©���Ļ�������ˮ������ƿ�У����Կ�����ƿ�г���ˮ������������
ˮ������������
��Һ�ηɽ�
Һ�ηɽ�
������ͬʱU�ιܲ���������Ϊ�״�Һ���½����Ҵ�Һ������
�״�Һ���½����Ҵ�Һ������
�������ʵ�������ǣ�Ϊ�˷�ֹ�����¹ʣ���ϡ��Ũ����ʱ��һ��Ҫ��
Ũ����
Ũ����
��������������ע��ˮ
ˮ
�У������Ͻ��裬�в�����ˮ����Ũ������
��ˮ����Ũ������
������������Ũ��������ʡ�ϡ�ͷ����Ƚ��з�����ɣ�
����⣺ˮ�ܶ�С��Ũ���ᣬ���µ�ˮ�θ���Ũ�����ϣ�Ũ��������ˮ�ų��������ȣ�ʹ����Ũ���������ˮ�������ڣ����Һ�ηɽ�������Ũ��������ˮ�ų��������ȣ�����ƿ�ڵ������������ͣ��ʼ�Һ���½���ʵ������ȷϡ��Ũ����ķ����ǣ���Ũ���������������ص���ˮ�У������ϵ��ò��������裬ʹ����Ѹ����ɢ���в��ɽ�ˮ����Ũ�����
�ʴ�Ϊ��ˮ�����������棻Һ�ηɽ����״�Һ���½����Ҵ�Һ��������Ũ���ˮ����ˮ����Ũ�����
�ʴ�Ϊ��ˮ�����������棻Һ�ηɽ����״�Һ���½����Ҵ�Һ��������Ũ���ˮ����ˮ����Ũ�����
�����������ѶȲ��Ǻܴ���Ҫ������Ũ��������ʺ�ϡ�ͷ���������ѧ����������������������

��ϰ��ϵ�д�
 �Ǽ�����������ϵ�д�
�Ǽ�����������ϵ�д� â���̸������Ծ�ϵ�д�
â���̸������Ծ�ϵ�д�
�����Ŀ
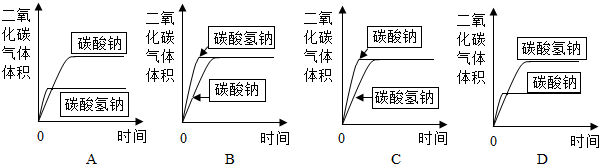
 9����ͼ��ʾ����ƿ��ʢ������X���ι���ʢ��Һ��Y������ѹ�ιܽ�ͷ��ʹҺ��Y����ƿ�У���һ����ɼ�С����a��������X��Һ��Y�������ǣ������� 9����ͼ��ʾ����ƿ��ʢ������X���ι���ʢ��Һ��Y������ѹ�ιܽ�ͷ��ʹҺ��Y����ƿ�У���һ����ɼ�С����a��������X��Һ��Y�������ǣ�������
|
��������AlN�������͵ĵ��Ӿ�Ե��Ƭ���ϣ����ڴ��ͺͳ���
�ͼ��ɵ�·�У��ڸ����µ�����̼��������������Ӧ�������ɵ������������һ����̼���ô˷����Ƶõĵ������к�����������̼����������ijУ��ѧ��ȤС������������Ӧԭ����ȡ���ⶨ������Ʒ�е�����������������
�������ϣ���1��Al2O3+2NaOH=2NaAlO2+H2O������2��AlN+NaOH+H2O=NaAlO2+NH3�������������ᷴӦ��
���ʵ�飺
ʵ��һ����ȡ��������Ʒ
ʵ������ⶨ���Ƶ�������Ʒ�е���������������
�ٰ�ͼ2��װ��������9.1g��������Ʒ������ƿ�У��ڷ�Һ©���м���һ������ŨNaOH��Һ
�ڳƵ�ʢ��ϡ������ձ���©����������Ϊ200g
�۴�ֹˮ��C���ӷ�Һ©������ƿ�е���ŨNaOH��Һ��ֱ�����ٲ�������Ϊֹ
�ܴ�ֹˮ��B���ӵ���A����������һ�����Ŀ���
���ٴγ���ʢ��ϡ������ձ���©����������Ϊ203.4g
���ظ�ʵ��ܢݲ����Ƶ�ʢ��ϡ������ձ���©������������Ϊ203.4g
ʵ�����ۣ���1������ټ�����Ʒǰ��Ӧ��� ��
��2��װ�м�ʯ�ҵĸ���������� ��װ����ʹ��©����Ŀ���� ��
��3��ͼ2�ձ���ϡ����ܻ���Ũ����������� ��ͼ2�ձ���ϡ����ܻ���Ũ����������� ��ͼ2�ձ���ϡ�����ܷ�ϡ���� ��
��4������ܵ�Ŀ���� ��
ʵ����ۣ����Ƶ�������Ʒ�е���������������Ϊ %��д������̣�4�֣���
�¹ʴ�����ʵ���У�С����С�Ľ�ʢ��Լ50mL��ŨNaOH��Һ���������ϣ���ʱ��Ӧ�ò�ȡ�Ĵ�ʩ�ǣ� ��
��չ̽������1��ʵ���ʣ���ŨNaOH��Һ ���ܻ��ܣ��Ż�ԭ�Լ�ƿ��д��NaOH��Һ¶���ڿ����з����Ļ�ѧ��Ӧ����ʽ ��
��2�����Ž�ԼҩƷ��ԭ��ͬʱ��Ҫȷ��ʵ��˳����óɹ�����ʵ���������Ӧ��40%ŨNaOH��Һ g��д������̣�4�֣���

�ͼ��ɵ�·�У��ڸ����µ�����̼��������������Ӧ�������ɵ������������һ����̼���ô˷����Ƶõĵ������к�����������̼����������ijУ��ѧ��ȤС������������Ӧԭ����ȡ���ⶨ������Ʒ�е�����������������
�������ϣ���1��Al2O3+2NaOH=2NaAlO2+H2O������2��AlN+NaOH+H2O=NaAlO2+NH3�������������ᷴӦ��
���ʵ�飺
ʵ��һ����ȡ��������Ʒ
| ʵ�鲽�� | ��ػ�ѧ��Ӧ����ʽ | ||||
| 1����ȥ�����з۳���������̼��������ٳ�ȥ������ | ��ȥ�����Ļ�ѧ��Ӧ����ʽΪ 2Cu+O2
| ||||
| 2���������Ƶõĵ���ͨ��װ��̼������������Ӳ�ʲ������и�����ȡ��������Ʒ����ͼ1���� | װ���з�����ѧ��Ӧ�Ļ�ѧ����ʽΪ N2+3C+Al2O3
|
�ٰ�ͼ2��װ��������9.1g��������Ʒ������ƿ�У��ڷ�Һ©���м���һ������ŨNaOH��Һ
�ڳƵ�ʢ��ϡ������ձ���©����������Ϊ200g
�۴�ֹˮ��C���ӷ�Һ©������ƿ�е���ŨNaOH��Һ��ֱ�����ٲ�������Ϊֹ
�ܴ�ֹˮ��B���ӵ���A����������һ�����Ŀ���
���ٴγ���ʢ��ϡ������ձ���©����������Ϊ203.4g
���ظ�ʵ��ܢݲ����Ƶ�ʢ��ϡ������ձ���©������������Ϊ203.4g
ʵ�����ۣ���1������ټ�����Ʒǰ��Ӧ���
��2��װ�м�ʯ�ҵĸ����������
��3��ͼ2�ձ���ϡ����ܻ���Ũ�����������
��4������ܵ�Ŀ����
ʵ����ۣ����Ƶ�������Ʒ�е���������������Ϊ
�¹ʴ�����ʵ���У�С����С�Ľ�ʢ��Լ50mL��ŨNaOH��Һ���������ϣ���ʱ��Ӧ�ò�ȡ�Ĵ�ʩ�ǣ�
��չ̽������1��ʵ���ʣ���ŨNaOH��Һ
��2�����Ž�ԼҩƷ��ԭ��ͬʱ��Ҫȷ��ʵ��˳����óɹ�����ʵ���������Ӧ��40%ŨNaOH��Һ


 30���������ƣ�Na2O2����һ�ֵ���ɫ���壮��һ�οƼ���У�ij��ѧ��ȤС���ͬѧ������Na2O2���������������ͼ1��ʵ���У������˸ߵͲ�ͬ���������������������Ϩ������������Ϩ��ͬʱҲ����ط�����ȼ����������
30���������ƣ�Na2O2����һ�ֵ���ɫ���壮��һ�οƼ���У�ij��ѧ��ȤС���ͬѧ������Na2O2���������������ͼ1��ʵ���У������˸ߵͲ�ͬ���������������������Ϩ������������Ϩ��ͬʱҲ����ط�����ȼ����������


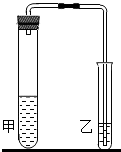 ��2012?��¥��һģ���о���ѧϰС���̼���ƺ�̼�����Ƶ����ʽ���̽�����������ʵ�飮
��2012?��¥��һģ���о���ѧϰС���̼���ƺ�̼�����Ƶ����ʽ���̽�����������ʵ�飮