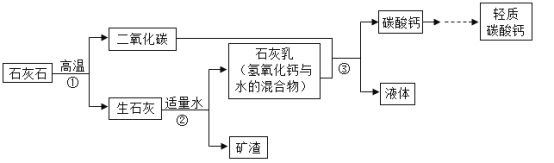
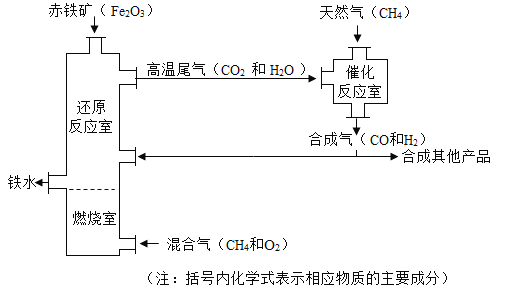
��Ŀ����
����Ŀ��ˮ������������������ϵ���С�
��1������ij��Ȼˮ��Ӳˮ������ˮ��ȡ�����������ˮ�����裬��ĭ�����а�ɫ��״�����Ȼˮ��_________��
��2����ͥ��Ӳˮת��Ϊ��ˮ�ķ���__________��
��3����ͼ1�ǵ��ˮ�ļ���װ��ͼ��
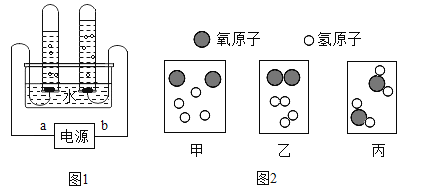
��ͼ1��Դ����a�ǵ�Դ��______��������������b�������Թ��������������______�����������ķ�����________
��4��������ͼ2����ʾˮ�ֽ�Ϊ���������������г��ֵ���ʾ��ͼ���밴�����ڻ�ѧ�仯�����г��ֵ�˳������______���üס��ҡ�����ʾ����
��5�����ˮ��Ӧ��ѧ����ʽΪ ___________
���𰸡�Ӳˮ ��� �� O2/���� �������ǵ�ľ�������Թ��ڸ�ȼ֤�������� �����ң��üס��ҡ�����ʾ�� 2 H2O ![]() 2H2��+O2��
2H2��+O2��
��������
��1������ij��Ȼˮ��Ӳˮ������ˮ��ȡ�����������ˮ�����裬��ĭ�����а�ɫ��״�˵�����н϶�ĸ�þ���ӣ�����Ȼˮ��Ӳˮ��
��2����ͥ��Ӳˮת��Ϊ��ˮ�ķ�������У���п�ʹ��þ���ӳ�����
��3������������������������һ�������a�˲�����������b�˵�2��������������a�ǵ�Դ�ĸ�������b�������Թ�����������������������������ķ����ǽ������ǵ�ľ�������Թ��ڸ�ȼ֤����������
��4���ɻ�ѧ��Ӧ��ʵ�ʿ�֪����ѧ��Ӧ�Ĺ��̾��Dzμӷ�Ӧ�ĸ����ʵ�ԭ�ӣ�������������������ʵĹ��̣����ԣ�ˮ�ֽ����������������Ĺ��̾���ˮ���ӷֽ�Ϊ��ԭ�Ӻ���ԭ�ӣ�ÿ������ԭ�ӹ�����һ������ӣ�ÿ������ԭ�ӹ���һ�������ӣ���ͼ�л�ѧ�仯�����г��ֵ�˳�����б����ס��ң��ñ仯�У���ԭ������ԭ�ӵ�����û�иı䣻��������ң�
��5�����ˮ������������������Ӧ����ʽΪ�� ��
��