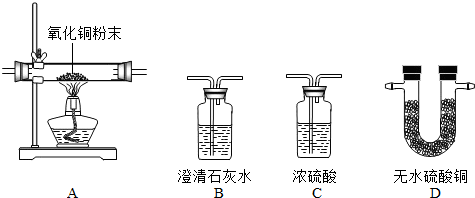
��Ŀ����
Ϊ��Ū��ij�����ķ��ն�������ɷ��Ƿ���ˮ������һ����̼�Ͷ�����̼���ס�������ʵ��С��ֱ�����ͼ��ʾʢ��ҩƷ��������ͼ�мг�������ʡ�ԣ�������ն��ڵ�����ɷ�
��1��������һ�����Ƿ�������ijһ���壬�����������Ƿ��ж�����̼Ӧѡ�ã� ���������ն��ں���ˮ��������B�е������ǣ� ������A�в������������ɺ�ɫ��ɺ�ɫ������ն��ں��У� ��
��2������������������װһ��װ�ã�ͨ��һ��ʵ��ͬʱ���������������Ƿ���ˮ������һ����̼�Ͷ�����̼��������ͨ�����Ⱥ�˳���������������ǣ�����ţ����������ظ�ʹ�ã��� ��
��3��������֤���ն��в�����һ����̼��������ն�ǰ��Ҫ���ж�����̼�����Ƿ�ϸߵ�ʵ�飬�䷽���ǣ� ��

��1��D��������CO
��2��B D C D A D E
��3���ƻ�ʵ��

��ϰ��ϵ�д�
�����Ŀ