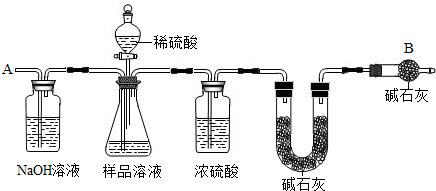
��Ŀ����
��֪ij������Ʒ�к��������Ȼ��ƣ�Ϊ�ⶨ��Ʒ�д���������������ס�������ֱ���ò�ͬ��������������ͼװ��ͨ���ⶨ���������ټ�������������������

����Ҫ�������£�
�ٰ�ͼ��װ��������������ԣ�
�ڳ�ȡm������������ƿ�м���������ˮʹ֮��ȫ�ܽ⣻
�۳���װ��D��װ�м�ʯ�ҵ�U�ι�������a1�ˣ�
�ܴӷ�Һ©���������μ�20%��ϡ����
�ݴӵ���a�л������������
���ٴγ���װ��D��װ�м�ʯ�ҵ�U�ι�������
���ظ��ݺ͢IJ�����ֱ��װ��D��U�ι������������䣬�������Ϊa2�ˣ�
�Իش�������⣺
��1��Aװ�������װ______��Һ��������______��
��2��װ��C��Ũ�����������______��
��3��������У��ӷ�Һ©���������μ�20%��ϡ����ֱ���۲쵽______Ϊֹ��д���÷�Ӧ�Ļ�ѧ����ʽ______��
��4����û�в���ݻ�ʹ�ⶨ���______��ƫ���ƫС����
��5��Eװ���м�ʯ�ҵ�������______��
��6��װ��B�з�Һ©���ڵ�ϡ����ܻ���Ũ�����������______��
�����������ɳ����ķ������ⶨ���Na2CO3����������������ȡ12.5����Ʒ����107.2�˵��Ȼ�����Һǡ����ȫ��Ӧ���˵õ��ij��������Ϊ19.7�˺�һ������Һ����û����ʧ�����Լ������Ʒ�д������������������Һ�����ʵ�����������
���𰸡���������1�����ݿ������ж�����̼��������Ҫ�����Ų�Ӱ��ʵ������
��2������װ�õ��ص㼰ʵ��Ŀ�ģ�����װ��C��Ũ��������ã�
��3�����������̼���Ʒ�Ӧ���ɶ�����̼������һ������н��
��4������װ�õ��ص㼰ʵ��Ŀ�ģ��������������һ������Ŀ�ģ�
��5����װ����װҩƷʱ��ɱ���Ļ���һЩ�������ݿ����ó��ɷ����������
��6������Ũ������лӷ��Խ��н��
����̼���ƺ��Ȼ�����Ӧ����̼�ᱵ��������̼�ᱵ���������̼���Ƶ������Լ���Ʒ�д����������������Һ�е�����Ϊ�Ȼ��ƣ������Һ�����ʵ�����������
����⣺��1���������֪������ͨ���ⶨ������̼���������ⶨ̼���Ƶ����������ģ�����Ҫ�ų������еĶ�����̼����ʵ����������A��װ�˼�����Һ�����տ����еĶ�����̼����Aװ�������װ������������Һ��Ŀ�ij�ȥ�����еĶ�����̼��
��2��Ũ���������ˮ�ԣ�װ��C��Ũ���������������ˮ�֣�
��3�������̼���Ʒ�Ӧ���ɶ�����̼���壬������У��ӷ�Һ©���������μ�20%��ϡ����ֱ���۲쵽����ð��Ϊֹ��˵����Ӧ������ϣ��÷�Ӧ�Ļ�ѧ����ʽ��Na2CO3+H2SO4=Na2SO4+H2O+CO2����
��4��Ϊ�˼�Сʵ��������ȡ�˹�������ķ������Ѳ�����װ��B�ж�����̼ȫ����D�м�ʯ�����գ�ʵ������ȷ����û�в���ݻ�ʹ������װ��B�ж�����̼��ȫ����D�м�ʯ�����գ��ⶨ���ƫС��
��5�����Dװ��ֱ��������������ͨ��������е�ˮ�����Ͷ�����̼�����Dװ�ö��Բⶨ�������Ӱ�죬����װ��E���������Ƿ�ֹ������ˮ�����Ͷ�����̼����װ��D�У�
��6��Ũ������лӷ��ԣ�����װ��B�з�Һ©���ڵ�ϡ����ܻ���Ũ����������ǣ�Ũ�����ӷ�ʹ������̼���Ȼ�������Ӱ��ʵ������
�⣺����Ʒ��̼���Ƶ�����Ϊx�������Ȼ��Ƶ�����Ϊy��
Na2CO3+BaCl2=BaCO3��+2NaCl
106 197 117
x 19.7g y

x=10.6g
��Ʒ�д������������= ×100%=84.8%
×100%=84.8%

y=11.7g
��Һ�����ʵ���������= ×100%=13.6%
×100%=13.6%
����Ʒ�д������������84.8%����Һ�����ʵ���������13.6%��
�ʴ�Ϊ����1���������ƣ���ȥ�����еĶ�����̼��
��2������ˮ�֣�
��3������ð�ݣ�Na2CO3+H2SO4=Na2SO4+H2O+CO2����
��4��ƫС��
��5����ֹ������CO2��ˮ��������D�У�
��6��Ũ�����ӷ�ʹ������̼���Ȼ�������Ӱ��ʵ������
��84.8%����13.6%��
������������Ҫ���������̼�ͼ�ʯ�ҵķ�Ӧ��ͨ����������Ҫ֪�������ʵ��ʱҪ�����ܵ��ų����ܶ�ʵ��������Ӱ������أ����籾���п����ж�����̼��ʵ������Ӱ�죬ˮ������Ӱ��ȣ�
��2������װ�õ��ص㼰ʵ��Ŀ�ģ�����װ��C��Ũ��������ã�
��3�����������̼���Ʒ�Ӧ���ɶ�����̼������һ������н��
��4������װ�õ��ص㼰ʵ��Ŀ�ģ��������������һ������Ŀ�ģ�
��5����װ����װҩƷʱ��ɱ���Ļ���һЩ�������ݿ����ó��ɷ����������
��6������Ũ������лӷ��Խ��н��
����̼���ƺ��Ȼ�����Ӧ����̼�ᱵ��������̼�ᱵ���������̼���Ƶ������Լ���Ʒ�д����������������Һ�е�����Ϊ�Ȼ��ƣ������Һ�����ʵ�����������
����⣺��1���������֪������ͨ���ⶨ������̼���������ⶨ̼���Ƶ����������ģ�����Ҫ�ų������еĶ�����̼����ʵ����������A��װ�˼�����Һ�����տ����еĶ�����̼����Aװ�������װ������������Һ��Ŀ�ij�ȥ�����еĶ�����̼��
��2��Ũ���������ˮ�ԣ�װ��C��Ũ���������������ˮ�֣�
��3�������̼���Ʒ�Ӧ���ɶ�����̼���壬������У��ӷ�Һ©���������μ�20%��ϡ����ֱ���۲쵽����ð��Ϊֹ��˵����Ӧ������ϣ��÷�Ӧ�Ļ�ѧ����ʽ��Na2CO3+H2SO4=Na2SO4+H2O+CO2����
��4��Ϊ�˼�Сʵ��������ȡ�˹�������ķ������Ѳ�����װ��B�ж�����̼ȫ����D�м�ʯ�����գ�ʵ������ȷ����û�в���ݻ�ʹ������װ��B�ж�����̼��ȫ����D�м�ʯ�����գ��ⶨ���ƫС��
��5�����Dװ��ֱ��������������ͨ��������е�ˮ�����Ͷ�����̼�����Dװ�ö��Բⶨ�������Ӱ�죬����װ��E���������Ƿ�ֹ������ˮ�����Ͷ�����̼����װ��D�У�
��6��Ũ������лӷ��ԣ�����װ��B�з�Һ©���ڵ�ϡ����ܻ���Ũ����������ǣ�Ũ�����ӷ�ʹ������̼���Ȼ�������Ӱ��ʵ������
�⣺����Ʒ��̼���Ƶ�����Ϊx�������Ȼ��Ƶ�����Ϊy��
Na2CO3+BaCl2=BaCO3��+2NaCl
106 197 117
x 19.7g y

x=10.6g
��Ʒ�д������������=
 ×100%=84.8%
×100%=84.8%
y=11.7g
��Һ�����ʵ���������=
 ×100%=13.6%
×100%=13.6%����Ʒ�д������������84.8%����Һ�����ʵ���������13.6%��
�ʴ�Ϊ����1���������ƣ���ȥ�����еĶ�����̼��
��2������ˮ�֣�
��3������ð�ݣ�Na2CO3+H2SO4=Na2SO4+H2O+CO2����
��4��ƫС��
��5����ֹ������CO2��ˮ��������D�У�
��6��Ũ�����ӷ�ʹ������̼���Ȼ�������Ӱ��ʵ������
��84.8%����13.6%��
������������Ҫ���������̼�ͼ�ʯ�ҵķ�Ӧ��ͨ����������Ҫ֪�������ʵ��ʱҪ�����ܵ��ų����ܶ�ʵ��������Ӱ������أ����籾���п����ж�����̼��ʵ������Ӱ�죬ˮ������Ӱ��ȣ�

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ