��Ŀ����
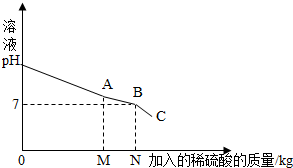 ��2013?���Ǹۣ�����֪����ÿ������ʵ���ʣ���Һ����ֱ���㵹�����۹ܣ�������մ�����ij��ͬѧʵ����ռ�������̼���ƺ��������ƵĻ�Ϸ�Һ5.11Kg��һ����ȤС���÷�����������������Ϊ19.6%�������������������մ��������Һ���������Һ�м���ϡ����ʱ��������ҺpH������ϡ���������Ĺ�ϵ������ͼ��ʾ����
��2013?���Ǹۣ�����֪����ÿ������ʵ���ʣ���Һ����ֱ���㵹�����۹ܣ�������մ�����ij��ͬѧʵ����ռ�������̼���ƺ��������ƵĻ�Ϸ�Һ5.11Kg��һ����ȤС���÷�����������������Ϊ19.6%�������������������մ��������Һ���������Һ�м���ϡ����ʱ��������ҺpH������ϡ���������Ĺ�ϵ������ͼ��ʾ������1��ͨ����ͼ��֪������Ӧ������ͼ��
B
B
��ʱ���A������B����C��������Һǡ�ô����꣨��̼���ƺ��������ƻ�Ϸ�Һ�պ���ȫת������������Һ������2��������Һ�м���ϡ������N��ʱ�������ϡ��������Ϊ5Kg����ʱ��Һ������Ϊ10Kg�����ʱ������Һ�����ʵ�����������д��������̣�����ˮ��������Բ��ƣ���
��������1������ͼ�������Bʱ����Һ��PH����7����Һ�����ԣ���Һǡ�ô����ꣻ
��2������̼���ƺ��������������ᷴӦ�ķ���ʽ���ҳ������������ƵĹ�ϵʽ�����������������������Ƶ��������ڸ�����Һ�����ʵ������������㣮
��2������̼���ƺ��������������ᷴӦ�ķ���ʽ���ҳ������������ƵĹ�ϵʽ�����������������������Ƶ��������ڸ�����Һ�����ʵ������������㣮
����⣺��1���������ɵ���������Һ�����ԣ���ͼʾ��֪������Ӧ������ͼ��B��ʱ��pH=7��Ȼ�����½���˵����ʱǡ�ý���Һ�����ꣻ
��2�������ɵ������Ƶ�����Ϊx
��H2SO4+2NaOH�TNa2SO4+2H2O��Na2CO3+H2SO4=Na2SO4+H2O+CO2��
�ã�H2SO4��Na2SO4
98 142
5Kg��19.6% x
=
��ã�x=1.42gKg
������Һ�����ʵ����������ǣ�
��100%=14.2%��
�𣺣�1��B����2��������Һ�����ʵ�����������14.2%��
��2�������ɵ������Ƶ�����Ϊx
��H2SO4+2NaOH�TNa2SO4+2H2O��Na2CO3+H2SO4=Na2SO4+H2O+CO2��
�ã�H2SO4��Na2SO4
98 142
5Kg��19.6% x
| 98 |
| 142 |
| 5Kg��19.6% |
| x |
��ã�x=1.42gKg
������Һ�����ʵ����������ǣ�
| 1.42Kg |
| 10Kg |
�𣺣�1��B����2��������Һ�����ʵ�����������14.2%��
�������ڽ������ʱ�����ȷ�����ӦӦ�õ�ԭ����Ȼ����ͼ���е�ת�۵㣬�Լ�����ʽ�еı�����ϵ���н��

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ