��Ŀ����
 26��A��B��C�����ֳ���������������£�A����ɫ��ζ�����壬B����ɫ��ζ��Һ�壬C��D��F�ǰ�ɫ���壬��Ȼ���к���F�Ŀ�ʯ����Ҫ�Ľ�������֮һ����E��D�еμ���ɫ��ʯ����Һ��E��ʯ��ʺ�ɫ��D��ʯ�����ɫ��B��C��������Dʱ���ų��������ȣ���ͼ��������ʵ�ת����ϵ��
26��A��B��C�����ֳ���������������£�A����ɫ��ζ�����壬B����ɫ��ζ��Һ�壬C��D��F�ǰ�ɫ���壬��Ȼ���к���F�Ŀ�ʯ����Ҫ�Ľ�������֮һ����E��D�еμ���ɫ��ʯ����Һ��E��ʯ��ʺ�ɫ��D��ʯ�����ɫ��B��C��������Dʱ���ų��������ȣ���ͼ��������ʵ�ת����ϵ���Իش�
��1���û�ѧʽ��ʾ�������ʣ�B
H2O
E
H2CO3
��2����д���б仯�Ļ�ѧ����ʽ
��
Ca��OH��2+CO2=CaCO3��+H2O
����
CaCO3+2HCl=CaCl2+H2O+CO2��
���������������֪��B��һ�ֳ�����������������ɫ��ζ��Һ�壬���Ƴ�B��H2O������Ȼ���к���F�Ŀ�ʯ����Ҫ�Ľ�������֮һ�����Ƴ�F��CaCO3����CaCO3��ϡ���ᷴӦ�õ�A��A��H2O��������E��E��ʯ��ʺ�ɫ�����Ƴ���A��CO2��E��H2CO3����D��ʯ�����ɫ��B��C��������Dʱ���ų��������ȣ�D��A��Ӧ����CaCO3�����Ƴ���C��CaO��D��Ca��OH��2��
����⣺��1���������֪��B��һ�ֳ�����������������ɫ��ζ��Һ�壬���Ƴ�B��H2O������Ȼ���к���F�Ŀ�ʯ����Ҫ�Ľ�������֮һ�����Ƴ�F��CaCO3��
��2���۲�ͼ��������֮���ת����ϵ��֪����CaCO3��ϡ���ᷴӦ�õ�A��A��H2O��������E��E��ʯ��ʺ�ɫ�����Ƴ���A��
CO2��E��H2CO3����D��ʯ�����ɫ��B��C��������Dʱ���ų��������ȣ�D��A��Ӧ����CaCO3�����Ƴ���C��CaO��D��Ca��OH��2��
�ʴ�Ϊ����1��H2O��H2CO3
��2��Ca��OH��2+CO2=CaCO3��+H2O��CaCO3+2HCl=CaCl2+H2O+CO2����
��2���۲�ͼ��������֮���ת����ϵ��֪����CaCO3��ϡ���ᷴӦ�õ�A��A��H2O��������E��E��ʯ��ʺ�ɫ�����Ƴ���A��
CO2��E��H2CO3����D��ʯ�����ɫ��B��C��������Dʱ���ų��������ȣ�D��A��Ӧ����CaCO3�����Ƴ���C��CaO��D��Ca��OH��2��
�ʴ�Ϊ����1��H2O��H2CO3
��2��Ca��OH��2+CO2=CaCO3��+H2O��CaCO3+2HCl=CaCl2+H2O+CO2����
���������������ƶ����е�ͼ���⣬��ͼ�ƶ������п��������ر���������ⷽʽ����������Ŀ�����ֶȸߣ��ʺ���ѧ�ͱ�ҵ�϶�Ϊһ�Ŀ�����ʽ������ѡ���˲ţ�������ۺ����ر�ǿ��������Ҫ��������к���ʵ�ػ���֪ʶ����Ҫ���нϸߵĽ��ⷽ����������

��ϰ��ϵ�д�
�����Ŀ
 27��A��B��C�����ֳ���������������£�A����ɫ��ζ�����壬B����ɫ��ζ��Һ�壬C��D��F�ǰ�ɫ���壬��Ȼ���к���F�Ŀ�ʯ����Ҫ�Ľ�������֮һ����E��D�еμ���ɫ��ʯ����Һ��E��ʯ��ʺ�ɫ��D��ʯ�����ɫ��B��C��������Dʱ���ų��������ȣ���ͼ��������ʵ�ת����ϵ��
27��A��B��C�����ֳ���������������£�A����ɫ��ζ�����壬B����ɫ��ζ��Һ�壬C��D��F�ǰ�ɫ���壬��Ȼ���к���F�Ŀ�ʯ����Ҫ�Ľ�������֮һ����E��D�еμ���ɫ��ʯ����Һ��E��ʯ��ʺ�ɫ��D��ʯ�����ɫ��B��C��������Dʱ���ų��������ȣ���ͼ��������ʵ�ת����ϵ�� ��2012?�ɶ���A��B��C�����ֳ����ĺ��ƻ��������֮������ͼ��ʾ��ת����ϵ�����ֲ�����ȥ����
��2012?�ɶ���A��B��C�����ֳ����ĺ��ƻ��������֮������ͼ��ʾ��ת����ϵ�����ֲ�����ȥ����
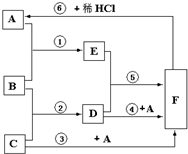 ��2007?�����ж�ģ��A��B��C�����ֳ���������������£�A����ɫ��ζ�����壬B����ɫ��ζ��Һ�壬C��D��F�ǰ�ɫ���壬��Ȼ���к���F�Ŀ�ʯ����Ҫ�Ľ�������֮һ����E��D�еμ���ɫ��ʯ����Һ��E��ʯ��ʺ�ɫ��D��ʯ�����ɫ��B��C��������Dʱ���ų��������ȣ���ͼ��������ʵ�ת����ϵ��
��2007?�����ж�ģ��A��B��C�����ֳ���������������£�A����ɫ��ζ�����壬B����ɫ��ζ��Һ�壬C��D��F�ǰ�ɫ���壬��Ȼ���к���F�Ŀ�ʯ����Ҫ�Ľ�������֮һ����E��D�еμ���ɫ��ʯ����Һ��E��ʯ��ʺ�ɫ��D��ʯ�����ɫ��B��C��������Dʱ���ų��������ȣ���ͼ��������ʵ�ת����ϵ��