��Ŀ����
ijУ���꼶��ѧʵ���������ķ������ɿ�����ǩȷ�����⣬С��ͬѧ�鵽����Ŀ�ǡ��Լ����Ǻ�ϡ����Ϊԭ����ȡCO2����С����ɸ�ʵ��IJ��ֲ���������ͼ��ʾ��
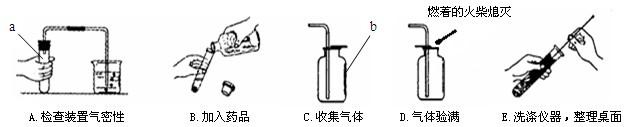
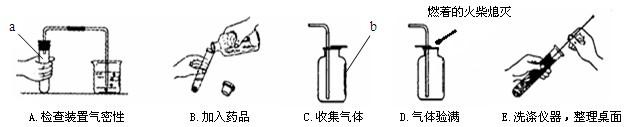
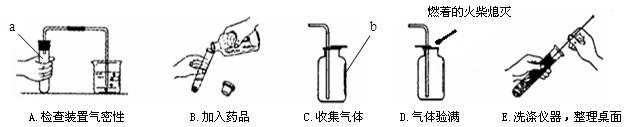
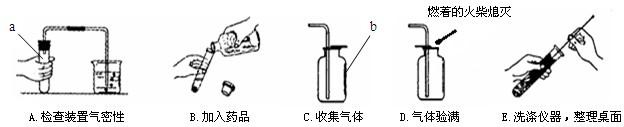
��1������a��b�����Ʒֱ���______��______��
��2��С���������������������Դ������______������ĸ��ţ���
��3������A�У�С���ɿ���Ƭ�̺����ձ��ڵĵ������γ�һ��______��֤��װ�ò�©����
��4��ʵ������ȡ������̼�Ļ�ѧ����ʽ��______�����������̼�ռ����ķ�����______��
��5�����������ǻ�Ϊп������������ʵ�����漰����������ҩƷ������ȡ��һ�ֳ������壬�÷�Ӧ�Ļ�ѧ����ʽ��______��
�⣺��1������a��b�����Ʒֱ��� �Թܡ�����ƿ��
��2��С���������������������Դ������B��ƿ��û�е��ţ���������Ⱦƿ���Լ���
��3������A�У������ձ��ڵĵ������γ�һ��ˮ����˵��ð�����ݺ�װ���ڵ���ѹ��С��֤��װ�ò�©����
��4��ʵ������ȡ������̼�Ļ�ѧ����ʽ�� CaCO3+2HCl=CaCl2+CO2��+H2O�����������̼�ռ����ķ����� ��ȼ�ŵĻ�����ƿ�ڣ�����Ϩ����˵��������̼�Ѿ��ռ�����
��5�����������ǻ�Ϊп������������ʵ�����漰����������ҩƷ������ȡ��һ�ֳ������壬�÷�Ӧ�Ļ�ѧ����ʽ�� Zn+2HCl=ZnCl2+H2��
�𰸣�����1���Թܡ�����ƿ��
��2��B��3��ˮ����4 CaCO3+2HCl=CaCl2+CO2��+H2O ��ȼ�ŵĻ�����ƿ�ڣ�����Ϩ����˵��������̼�Ѿ��ռ�����
��5��Zn+2HCl=ZnCl2+H2��
������ʵ������ȡ������̼�Ļ�������Ϊ��1�����װ�������� 2��װ��ҩƷ���ȼ�ʯ��ʯ���ټ�ϡ���ᣮ3����Ҫ��װ������� 4���ռ�������̼������ 5��ϴ���������������森ÿһ������������Ҫע���������������ʱ˫����ס�Թܣ�����������ð�����������γ�ˮ����˵�����������ã�װ��ҩƷʱҪ��Ҫ��������룬ƿ��Ҫ���ţ���ǩ�������ģ��ռ�ʱ����Ҫ���뼯��ƿ�ײ�������ʱľ��Ҫ���ڼ���ƿ�ڣ�
���������ʵ������ȡ��Ҫ�����ʵ�鲽�裬����ȷÿ�������е�ע�����
��2��С���������������������Դ������B��ƿ��û�е��ţ���������Ⱦƿ���Լ���
��3������A�У������ձ��ڵĵ������γ�һ��ˮ����˵��ð�����ݺ�װ���ڵ���ѹ��С��֤��װ�ò�©����
��4��ʵ������ȡ������̼�Ļ�ѧ����ʽ�� CaCO3+2HCl=CaCl2+CO2��+H2O�����������̼�ռ����ķ����� ��ȼ�ŵĻ�����ƿ�ڣ�����Ϩ����˵��������̼�Ѿ��ռ�����
��5�����������ǻ�Ϊп������������ʵ�����漰����������ҩƷ������ȡ��һ�ֳ������壬�÷�Ӧ�Ļ�ѧ����ʽ�� Zn+2HCl=ZnCl2+H2��
�𰸣�����1���Թܡ�����ƿ��
��2��B��3��ˮ����4 CaCO3+2HCl=CaCl2+CO2��+H2O ��ȼ�ŵĻ�����ƿ�ڣ�����Ϩ����˵��������̼�Ѿ��ռ�����
��5��Zn+2HCl=ZnCl2+H2��
������ʵ������ȡ������̼�Ļ�������Ϊ��1�����װ�������� 2��װ��ҩƷ���ȼ�ʯ��ʯ���ټ�ϡ���ᣮ3����Ҫ��װ������� 4���ռ�������̼������ 5��ϴ���������������森ÿһ������������Ҫע���������������ʱ˫����ס�Թܣ�����������ð�����������γ�ˮ����˵�����������ã�װ��ҩƷʱҪ��Ҫ��������룬ƿ��Ҫ���ţ���ǩ�������ģ��ռ�ʱ����Ҫ���뼯��ƿ�ײ�������ʱľ��Ҫ���ڼ���ƿ�ڣ�
���������ʵ������ȡ��Ҫ�����ʵ�鲽�裬����ȷÿ�������е�ע�����

��ϰ��ϵ�д�
 ���Ӣ��������ϵ�д�
���Ӣ��������ϵ�д�
�����Ŀ