��Ŀ����
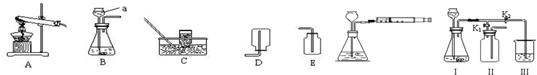
ij��ѧ�о���ѧϰС���ͬѧ����������Ȥζʵ�飬װ������ͼ(����������)����������Һ©����������Һ�����ʢ�й�����Թ�2��ʱ���۲쵽��ͬ������

��1�����Թ�2�з����˻�ѧ��Ӧ���۲쵽�Թ�1�������ݲ����������е�ʯ����Һ��죬�Թ�2�з�����Ӧ�Ļ�ѧ����ʽΪ ��
��2�����Թ�2��û�з�����ѧ��Ӧ���۲쵽��֧���ܿ�ͬʱð
���ݡ�����ƿ�ڵ�ʯ��ˮ����ǣ����Թ�2�еĹ��������____________��
��3�����۲쵽����ƿ��ʢ�еĺ�ɫ��Һ����ɫ����Һ©���е�Һ�塢�Թ�2�еĹ���ֱ���____________���ձ��е�Һ�������__________�����������Һ��ɫ��ԭ��_______________��
���𰸡�
��1��CaCO3 + 2HCl ====CaCl 2+ H2O + CO2��(̼�������ᷴӦ���ɶ�����̼����)
��2��NaOH ���ռ�������ƹ��壩
��3��ˮ������泥���ɱ������� �ᡣ
�Թ�2�еĹ����ܽ⣨����Һ�巴Ӧ��ʱ����������ʹ����ƿ�е�ѹǿ��С���ձ��е��ᵹ������ƿ�У�����ƿ�м������ʷ�����Ӧ��ʹ��ɫ��Һ����ɫ��
��1.˵���Թ�2�н��£� 2.˵������ƿ��ѹǿ��С��3.���к͵����ԣ�
����������

��ϰ��ϵ�д�
 53���ò�ϵ�д�
53���ò�ϵ�д�
�����Ŀ
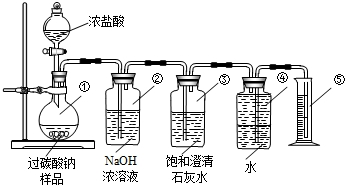
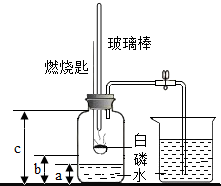 ij��ѧ�о���ѧϰС����ѧϰ�ˡ����������������ⶨ��ʵ��Ļ����ϣ��Ľ��˽̲��е�ʵ�飬��Ƴ���ͼ��ʾ��ʵ��װ�ã�
ij��ѧ�о���ѧϰС����ѧϰ�ˡ����������������ⶨ��ʵ��Ļ����ϣ��Ľ��˽̲��е�ʵ�飬��Ƴ���ͼ��ʾ��ʵ��װ�ã�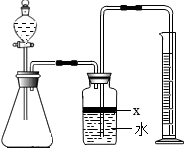 ��������AlN����һ�����������ϣ��㷺Ӧ���ڼ��ɵ�·��������ij�������к���̼�����������ʣ�ij��ѧ�о���ѧϰС����Ʋ����������ʵ�飮
��������AlN����һ�����������ϣ��㷺Ӧ���ڼ��ɵ�·��������ij�������к���̼�����������ʣ�ij��ѧ�о���ѧϰС����Ʋ����������ʵ�飮