��Ŀ����
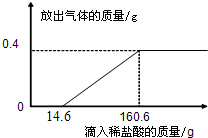
��ѧ��ȤС�������������һ����̼���ڸ���������Ӧ��ԭ�������ڲⶨijЩ��������Ʒ������������������������С��ͬѧȡ����������Ʒ10.425g����������һ����̼��ַ�Ӧ���õ�������4%�Ľ�����5.625g���������������е����ʲ��μӷ�Ӧ��
��1�������������������һ����̼��Ӧ�Ļ�ѧ����ʽ��______��
��2��ͨ�������������������Ʒ������������������������
�⣺��1������һ����̼�ܹ���ԭ�������������Ͷ�����̼�����Դ˻�ѧ����ʽΪ��3CO+Al2O3 2Al+3CO2��
2Al+3CO2��
��2���������������������������x
3CO+Al2O3 2Al+3CO2
2Al+3CO2
102 54
x 5.625g����1-4%��

x=10.2g
���Ը���������Ʒ����������������������Ϊ�� ��100%��97.8%��
��100%��97.8%��
�ʴ�Ϊ����1��3CO+Al2O3 2Al+3CO2����2������������Ʒ����������������������Ϊ97.8%��
2Al+3CO2����2������������Ʒ����������������������Ϊ97.8%��
��������1������һ����̼�ܹ���ԭ�������������Ͷ�����̼��д����ʽ���ɣ�
��2���������ɵĴ���������ͨ���÷�Ӧ�ķ���ʽ��������������������������������������������Ʒ������������������������
�����������ǶԺ��������ʵĻ�ѧ����ʽ���㿼���⣬����Ĺؼ��������йػ�ѧ����ʽ����ʱ��������ʵ�����һ���Ǵ���������������ܺ������ʣ�
 2Al+3CO2��
2Al+3CO2����2���������������������������x
3CO+Al2O3
 2Al+3CO2
2Al+3CO2102 54
x 5.625g����1-4%��

x=10.2g
���Ը���������Ʒ����������������������Ϊ��
 ��100%��97.8%��
��100%��97.8%���ʴ�Ϊ����1��3CO+Al2O3
 2Al+3CO2����2������������Ʒ����������������������Ϊ97.8%��
2Al+3CO2����2������������Ʒ����������������������Ϊ97.8%����������1������һ����̼�ܹ���ԭ�������������Ͷ�����̼��д����ʽ���ɣ�
��2���������ɵĴ���������ͨ���÷�Ӧ�ķ���ʽ��������������������������������������������Ʒ������������������������
�����������ǶԺ��������ʵĻ�ѧ����ʽ���㿼���⣬����Ĺؼ��������йػ�ѧ����ʽ����ʱ��������ʵ�����һ���Ǵ���������������ܺ������ʣ�

��ϰ��ϵ�д�
�����Ŀ
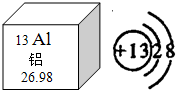 ������Ϊ������Ľ������������绯ѧʷ���أ�����������1825��ű�Ӣ����ѧ�Ҵ�ά�Ƶã����죬���Ѿ����������������ÿһ�����䣮
������Ϊ������Ľ������������绯ѧʷ���أ�����������1825��ű�Ӣ����ѧ�Ҵ�ά�Ƶã����죬���Ѿ����������������ÿһ�����䣮