��Ŀ����
��C��H��O��N��S��P��Cl��Na����Ca��Fe��K��Ag��Cu��13��Ԫ����ѡ��ǡ����Ԫ�أ������ӷ��š���ѧʽ��ѧ����ʽ������Ҫ����գ�
��1��3��̼���������_________
��2������������Ԫ����+5��______
��3��������Ԫ�ص�����ȼ����__________��
��4������Ԫ�صĵ�����______��
��5��ʵ������ȡ������̼___________
��6���кͷ�Ӧ______________
��7����������μӵĻ�ԭ��Ӧ_____________
��8���е��������ɵ��û���Ӧ_____________
��9���мط����ɵĸ��ֽⷴӦ_____________
��1��3��̼���������_________
��2������������Ԫ����+5��______
��3��������Ԫ�ص�����ȼ����__________��
��4������Ԫ�صĵ�����______��
��5��ʵ������ȡ������̼___________
��6���кͷ�Ӧ______________
��7����������μӵĻ�ԭ��Ӧ_____________
��8���е��������ɵ��û���Ӧ_____________
��9���мط����ɵĸ��ֽⷴӦ_____________
��1��3HCO3-
��2��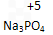
��3��C2H5OH
��4����NH4��2SO4
��5��CaCO3+2HCl==CaCl2+H2O+CO2����
��6��NaOH+HCl==NaCl+H2O
��7��Fe2O3+3CO 2Fe+3CO2
2Fe+3CO2
��8��Cu+2AgNO3=Cu(NO3)2+2Ag
��9��AgNO3+KCl==AgCl��+KNO3
��2��
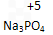
��3��C2H5OH
��4����NH4��2SO4
��5��CaCO3+2HCl==CaCl2+H2O+CO2����
��6��NaOH+HCl==NaCl+H2O
��7��Fe2O3+3CO
 2Fe+3CO2
2Fe+3CO2��8��Cu+2AgNO3=Cu(NO3)2+2Ag
��9��AgNO3+KCl==AgCl��+KNO3

��ϰ��ϵ�д�
 ���������ּ���ÿһ��ȫ�º�����ҵ��ϵ�д�
���������ּ���ÿһ��ȫ�º�����ҵ��ϵ�д� ��ٽ������½������������ϵ�д�
��ٽ������½������������ϵ�д�
�����Ŀ