��Ŀ����
�ֽ�25.3g��������KNO3���ʵ�Na2CO3��ĩ��Ʒ��������ƿ�м�����ˮ���Ƴ���Һ����������μ���δ֪��������������CaCl2��Һֱ����������������ƿ����Һ����������Һ��NaCl����������Ĺ�ϵ��ͼ��ʾ��
����
��1����CaCl2��Һ��Na2CO3��Һǡ����ȫ��Ӧʱ��������Һ����������
 ��2��Na2CO3��ĩ��Ʒ�Ĵ��ȣ�
��2��Na2CO3��ĩ��Ʒ�Ĵ��ȣ�
��3���μӵ�CaCl2��Һ����������������
���������������һλС����
�⣺��1�����Ȼ�����Һ�ļ��룬��Һ���Ȼ��������ӣ�����ȫ��Ӧʱ�Ȼ������������ֵ������������Ȼ����������������ֵʱ����Ӧ����Һ������CaCl2��Һ��Na2CO3��Һǡ����ȫ��Ӧʱ������Һ�������������߲�ã���ʱ��Һ����Ϊ100g��
��2������Ʒ��̼���Ƶ�����Ϊx
Na2CO3+CaCl2�TCaCO3��+2NaCl
106 117
x 100g��27.4%
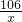 =
= x=24.8
x=24.8
Na2CO3��ĩ��Ʒ�Ĵ���= ��100%=98.0%
��100%=98.0%
��3�������߿ɵ�֪����ǡ����ȫ��Ӧʱ��Һ������������100g-60g=40g
�跴Ӧ����̼��Ƶ�����Ϊy�������Ȼ��Ƶ�����Ϊz
Na2CO3+CaCl2�TCaCO3��+2NaCl
111 100 117
z y 100g��27.4%
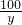 =
= =
=
y=23.4g��z=26g
�������Ȼ��Ƶ�����=100g+23.4g-60g=63.4g
�μӵ�CaCl2��Һ��������������= ��100%=41.0%
��100%=41.0%
�𣺣�1����CaCl2��Һ��Na2CO3��Һǡ����ȫ��Ӧʱ��������Һ��������Ϊ100g��
��2��Na2CO3��ĩ��Ʒ�Ĵ���Ϊ98.0%��
��3���μӵ�CaCl2��Һ��������������Ϊ41.0%��
��������1���������⣬������ƿ����Һ����������Һ��NaCl����������Ĺ�ϵͼ���ɵ�֪��Һ��NaCl�����������ﵽ���ֵʱ��CaCl2��Һ��Na2CO3��Һǡ����ȫ��Ӧ���������߿ɲ��������Һ����������
��2����ǡ����ȫ��Ӧʱ�����Ȼ��Ƶ�����������̼�������Ȼ��Ʒ�Ӧ�Ļ�ѧ����ʽ���ɼ����ĩ��Ʒ������̼���Ƶ��������ɴ����Na2CO3��ĩ��Ʒ�Ĵ��ȣ�
��3�����ݷ�Ӧ�Ļ�ѧ����ʽ���������Ȼ��������ɼ��������Ȼ�����Һ���Ȼ��Ƶ�������Ȼ�����������غ㶨���ټ�����������Ȼ�����Һ������������õμӵ�CaCl2��Һ����������������
�������������в���μ��Ȼ�����Һ�뷴Ӧ����Һ��������ϵ�����ֳ������Ϣ�봦����Ϣ�ķ���������
��2������Ʒ��̼���Ƶ�����Ϊx
Na2CO3+CaCl2�TCaCO3��+2NaCl
106 117
x 100g��27.4%
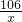 =
= x=24.8
x=24.8Na2CO3��ĩ��Ʒ�Ĵ���=
 ��100%=98.0%
��100%=98.0%��3�������߿ɵ�֪����ǡ����ȫ��Ӧʱ��Һ������������100g-60g=40g
�跴Ӧ����̼��Ƶ�����Ϊy�������Ȼ��Ƶ�����Ϊz
Na2CO3+CaCl2�TCaCO3��+2NaCl
111 100 117
z y 100g��27.4%
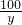 =
= =
=
y=23.4g��z=26g
�������Ȼ��Ƶ�����=100g+23.4g-60g=63.4g
�μӵ�CaCl2��Һ��������������=
 ��100%=41.0%
��100%=41.0%�𣺣�1����CaCl2��Һ��Na2CO3��Һǡ����ȫ��Ӧʱ��������Һ��������Ϊ100g��
��2��Na2CO3��ĩ��Ʒ�Ĵ���Ϊ98.0%��
��3���μӵ�CaCl2��Һ��������������Ϊ41.0%��
��������1���������⣬������ƿ����Һ����������Һ��NaCl����������Ĺ�ϵͼ���ɵ�֪��Һ��NaCl�����������ﵽ���ֵʱ��CaCl2��Һ��Na2CO3��Һǡ����ȫ��Ӧ���������߿ɲ��������Һ����������
��2����ǡ����ȫ��Ӧʱ�����Ȼ��Ƶ�����������̼�������Ȼ��Ʒ�Ӧ�Ļ�ѧ����ʽ���ɼ����ĩ��Ʒ������̼���Ƶ��������ɴ����Na2CO3��ĩ��Ʒ�Ĵ��ȣ�
��3�����ݷ�Ӧ�Ļ�ѧ����ʽ���������Ȼ��������ɼ��������Ȼ�����Һ���Ȼ��Ƶ�������Ȼ�����������غ㶨���ټ�����������Ȼ�����Һ������������õμӵ�CaCl2��Һ����������������
�������������в���μ��Ȼ�����Һ�뷴Ӧ����Һ��������ϵ�����ֳ������Ϣ�봦����Ϣ�ķ���������

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ

 ��2��Na2CO3��ĩ��Ʒ�Ĵ��ȣ�
��2��Na2CO3��ĩ��Ʒ�Ĵ��ȣ�
