��Ŀ����
��ϰʱ������ר���̽���ķ���ѧϰ�������ʵ���ɡ���������ȡ����������ʦ�������ǻع��˼���̽��ʵ�飮��1��̽��1���о�������������-���������������Ƿ�պ���ȫ�кͣ�
��̽�����ò�����պȡ��Ӧ�����Һ����pH��ֽ�ϣ�����pH��7��˵�����������
����֤��
| ʵ������ | ʵ������ | ��������� |
| ȡ������Һ���Թ��У� | ֤���о�������ȷ |
��2��̽��2���о��������ʵ���ȡ-��ȡ����������Һ��
��ͬѧ��ʢ�г���ʯ��ˮ���ձ��м��������̼������Һ�����ɰ�ɫ���������˺�õ���ɫ��Һ����˵�������Ƶ�������������Һ����
��ͬѧȴ˵����������ɫ��Һ��һ�������������ơ������������ʵ�飺
| ʵ������ | ʵ������ | ʵ����� |
| ���Թ�ȡ��ɫ��Һ����������2�η�̪��Һ | ��ɫ��̪��� | ��ɫ��Һ������������ |
��ͬѧ�������һ��ʵ�飺
| ʵ�鲽�� | ʵ������ | ʵ����� |
| ��ɫ��Һ��ȷʵ������������ |
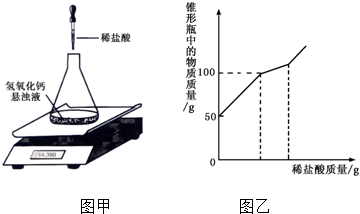
��3������ʵ�鷢�֣�ijͬѧȡ53g 10%��̼������Һ��152g���ͳ���ʯ��ˮǡ����ȫ��Ӧ����Ӧ����Һ�����ʵ�����������
���𰸡�����������̽��1����������е���Ϣ������������ʱ��Ҫ��������Ļ�ѧ���ʣ���֤������Ĵ��ڣ�
����̽��2������Ҫ���ǵ�̼���Ƶ�ˮ��Һ�ʼ��ԣ�����֤�������ƵĴ���ʱҪ�ų�̼���Ƶĸ���
̽��2 �е����һ���ڼ���ʱҪע�ⷴӦ����Һ��������m��=��Ӧǰ��������ʵ������ܺ�-���ܵ����ʵ�����-���ɵij���������-���ɵ����������
����⣨1��������pH��7�����������Ҫ��֤����������ǵ�����Ļ�ѧ�����ܺͻ��ý�����Ӧ�����������������ԣ�
���������м���п������֤����Ĵ��ڣ��ʴ�Ϊ���ټ���п�� �����ݲ��� ��Zn+2HCl=ZnCl2+H2�����𰸺������ɣ�
��2�����������֪��̼������Һʱ�����ģ���̼������Һ�ʼ��ԣ���ͬѧû�п��ǵ���һ�㣬��������ʵ�鷽���������ܣ�
�ʴ�Ϊ��̼������ҺҲ����ʹ��̪��죨��̼������ҺҲ�Ǽ��Եģ�
��3��Ҫ֤����ͬѧ�Ľ�����ȷ����Ӧ�����ų�̼���Ƶĸ��ţ�Ȼ�������֤�������ƵĴ��ڣ���������Ӧ���ȳ�ȥ̼���ƣ���ȥ̼���Ƶķ����кܶ��֣����õķ����ľ��ǰ���ת��Ϊ�������������壮
�ʴ�Ϊ��ȡ������Һ�������м���������BaCl2��Һ�����ˣ�Ȼ������Һ�м����̪������������Ҳ�ɣ�
û��֤�������������� �ų���̼���Ƶĸ��� ֤�����������ƵĴ���
��4���⣺�����ɵ�������������Ϊx�����ɵ�̼��Ƶ�����Ϊy��
Na2CO3+Ca��OH��2=CaCO3��+2NaOH
106 100 80
53g×10% y x
=5.3g


��� x=4g y=5g
��NaOH= =2%
=2%
�𣺷�Ӧ��������Һ����������Ϊ2%��
����������Ϊ�ۺϵ�ʵ��̽���⣬����Ӧ����������˼·�������ж�����е���Ϣ������ϵ������ʵ������ر��ǻ�ѧ�����������Ŀ
����̽��2������Ҫ���ǵ�̼���Ƶ�ˮ��Һ�ʼ��ԣ�����֤�������ƵĴ���ʱҪ�ų�̼���Ƶĸ���
̽��2 �е����һ���ڼ���ʱҪע�ⷴӦ����Һ��������m��=��Ӧǰ��������ʵ������ܺ�-���ܵ����ʵ�����-���ɵij���������-���ɵ����������
����⣨1��������pH��7�����������Ҫ��֤����������ǵ�����Ļ�ѧ�����ܺͻ��ý�����Ӧ�����������������ԣ�
���������м���п������֤����Ĵ��ڣ��ʴ�Ϊ���ټ���п�� �����ݲ��� ��Zn+2HCl=ZnCl2+H2�����𰸺������ɣ�
��2�����������֪��̼������Һʱ�����ģ���̼������Һ�ʼ��ԣ���ͬѧû�п��ǵ���һ�㣬��������ʵ�鷽���������ܣ�
�ʴ�Ϊ��̼������ҺҲ����ʹ��̪��죨��̼������ҺҲ�Ǽ��Եģ�
��3��Ҫ֤����ͬѧ�Ľ�����ȷ����Ӧ�����ų�̼���Ƶĸ��ţ�Ȼ�������֤�������ƵĴ��ڣ���������Ӧ���ȳ�ȥ̼���ƣ���ȥ̼���Ƶķ����кܶ��֣����õķ����ľ��ǰ���ת��Ϊ�������������壮
�ʴ�Ϊ��ȡ������Һ�������м���������BaCl2��Һ�����ˣ�Ȼ������Һ�м����̪������������Ҳ�ɣ�
û��֤�������������� �ų���̼���Ƶĸ��� ֤�����������ƵĴ���
��4���⣺�����ɵ�������������Ϊx�����ɵ�̼��Ƶ�����Ϊy��
Na2CO3+Ca��OH��2=CaCO3��+2NaOH
106 100 80
53g×10% y x
=5.3g


��� x=4g y=5g
��NaOH=
 =2%
=2%�𣺷�Ӧ��������Һ����������Ϊ2%��
����������Ϊ�ۺϵ�ʵ��̽���⣬����Ӧ����������˼·�������ж�����е���Ϣ������ϵ������ʵ������ر��ǻ�ѧ�����������Ŀ

��ϰ��ϵ�д�
�����Ŀ
��ϰʱ������ר���̽���ķ���ѧϰ�������ʵ���ɡ���������ȡ����������ʦ�������ǻع��˼���̽��ʵ�飮
��1��̽��1���о�������������-���������������Ƿ�պ���ȫ�кͣ�
��̽�����ò�����պȡ��Ӧ�����Һ����pH��ֽ�ϣ�����pH��7��˵�����������
����֤��
д�������漰�Ļ�ѧ����ʽ
��2��̽��2���о��������ʵ���ȡ-��ȡ����������Һ��
��ͬѧ��ʢ�г���ʯ��ˮ���ձ��м��������̼������Һ�����ɰ�ɫ���������˺�õ���ɫ��Һ����˵�������Ƶ�������������Һ����
��ͬѧȴ˵����������ɫ��Һ��һ�������������ơ������������ʵ�飺
��ͬѧ��Ϊ��ͬѧʵ�鷽��Ҳ�������ܣ�����֤����ɫ��Һ��һ�������������ƣ������� ��
��ͬѧ�������һ��ʵ�飺
����Ϊ����ͬѧ�ͱ�ͬѧ��ʵ����Ϊ����֤��ͬѧ���۵Ŀɿ��ԣ���ͬѧ�Ľ��۲��ɿ���ԭ���� ����Ȼ��ͬѧ��ʵ��ɿ��Ա���ͬѧ��ʵ��ã�ԭ���� ��
��3������ʵ�鷢�֣�ijͬѧȡ53g 10%��̼������Һ��152g���ͳ���ʯ��ˮǡ����ȫ��Ӧ����Ӧ����Һ�����ʵ�����������
��1��̽��1���о�������������-���������������Ƿ�պ���ȫ�кͣ�
��̽�����ò�����պȡ��Ӧ�����Һ����pH��ֽ�ϣ�����pH��7��˵�����������
����֤��
| ʵ������ | ʵ������ | ��������� |
| ȡ������Һ���Թ��У� | ֤���о�������ȷ |
��2��̽��2���о��������ʵ���ȡ-��ȡ����������Һ��
��ͬѧ��ʢ�г���ʯ��ˮ���ձ��м��������̼������Һ�����ɰ�ɫ���������˺�õ���ɫ��Һ����˵�������Ƶ�������������Һ����
��ͬѧȴ˵����������ɫ��Һ��һ�������������ơ������������ʵ�飺
| ʵ������ | ʵ������ | ʵ����� |
| ���Թ�ȡ��ɫ��Һ����������2�η�̪��Һ | ��ɫ��̪��� | ��ɫ��Һ������������ |
��ͬѧ�������һ��ʵ�飺
| ʵ�鲽�� | ʵ������ | ʵ����� |
| ��ɫ��Һ��ȷʵ������������ |
��3������ʵ�鷢�֣�ijͬѧȡ53g 10%��̼������Һ��152g���ͳ���ʯ��ˮǡ����ȫ��Ӧ����Ӧ����Һ�����ʵ�����������