��Ŀ����
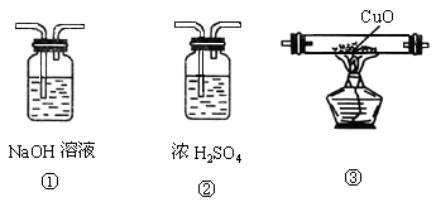
���л�������������̼��һ����̼���壬ijͬѧ���ô����������һ����̼��ԭ����ͭ������֤��Ӧ����������ͼ��ʾװ�ûش𣺣�����A����ͼ��ʾʢ��CO2��CO������������ƿ��ŨH2SO4������ˮ�ԣ�

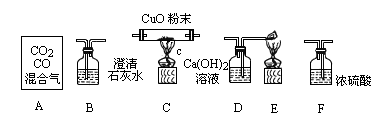
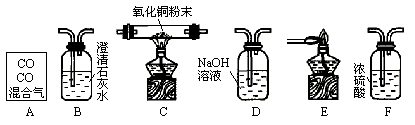
(1 )������ĸ��ʾװ�����ӵ���ȷ˳��ÿ��װ��ֻ��ʹ��һ�Σ���A�� ______________��
(2)��Cװ���й۲쵽��������___________________����Eװ���й۲쵽��������_______________________________��
(3)Bװ�õ�������_____________________����Ӧ�Ļ�ѧ����ʽΪ ____________________��
(4)Dװ�õ�������____________________________��
(5)д������װ���з����Ļ��Ϸ�Ӧ�ķ���ʽ��________________________ ��
(2)��Cװ���й۲쵽��������___________________����Eװ���й۲쵽��������_______________________________��
(3)Bװ�õ�������_____________________����Ӧ�Ļ�ѧ����ʽΪ ____________________��
(4)Dװ�õ�������____________________________��
(5)д������װ���з����Ļ��Ϸ�Ӧ�ķ���ʽ��________________________ ��
(1) B��F��C��D��E
(2)��ɫ��CuO��ĩ��ɺ�ɫ��������ɫ����
(3)��ȥһ����̼�еĶ�����̼��CO2+ Ca(OH)2=CaCO3��+H2O
(4)��֤CO��ԭCuO�IJ���
(5) 2CO+ O2 2CO2
2CO2
(2)��ɫ��CuO��ĩ��ɺ�ɫ��������ɫ����
(3)��ȥһ����̼�еĶ�����̼��CO2+ Ca(OH)2=CaCO3��+H2O
(4)��֤CO��ԭCuO�IJ���
(5) 2CO+ O2
 2CO2
2CO2
��ϰ��ϵ�д�
�����Ŀ