��Ŀ����
 ��֪ij������ĩ�г�����Al�����һ������Fe��Cu��Ϊ�ⶨ����Al������������ij��ѧ��ȤС���ͬѧչ�������µ�ʵ��̽�����������ߣ�Al������������Һ��Ӧ��������ˮ��ƫ�����ƺ���������Ӧ�Ļ�ѧ����ʽΪ��2A1+2H2O+2NaOH�T2NaAlO2+3H2������Fe��Cu��������������Һ��Ӧ��
��֪ij������ĩ�г�����Al�����һ������Fe��Cu��Ϊ�ⶨ����Al������������ij��ѧ��ȤС���ͬѧչ�������µ�ʵ��̽�����������ߣ�Al������������Һ��Ӧ��������ˮ��ƫ�����ƺ���������Ӧ�Ļ�ѧ����ʽΪ��2A1+2H2O+2NaOH�T2NaAlO2+3H2������Fe��Cu��������������Һ��Ӧ��ȡ20g�ý�����ĩ����100g����������Һƽ���ֳ�5�����μ��룬��ַ�Ӧ���˳����壬����ϴ�ӡ����������ʵ������еõ��IJ���������ͼ��
| ��NaOH��Һ�Ĵ��� | ��һ�� | �ڶ��� | ������ | �� |
| ��������ʣ����������/g | 16.5 | n | 9.5 | �� |
��2���ý�����ĩ��Al����������Ϊ
��3����ʽ���㣺��������������Һ��������������Ϊ���٣�����ȷ��0.1%����
��������1�������������ݿ�֪����һ�η�Ӧ��ȥ����3.5g���壬�ڶ��Σ�ҲӦ��Ӧ������3.5g���ҳ����ɣ�ÿ�μ��ٹ���3.5g�������ϱ���n��ֵΪ13��
��2����ͼ�п�֪��ʣ������Ϊ����Ϊ6g������������Ϊ20g-6g����ý�����ĩ��Al������������
��3�����û�ѧ����ʽ�Ľ��м��㣺������������Һ��������������Ϊx����ȷ��д��ѧ����ʽ��������֪����δ֪�����б���ʽ������������������Һ����������������
��2����ͼ�п�֪��ʣ������Ϊ����Ϊ6g������������Ϊ20g-6g����ý�����ĩ��Al������������
��3�����û�ѧ����ʽ�Ľ��м��㣺������������Һ��������������Ϊx����ȷ��д��ѧ����ʽ��������֪����δ֪�����б���ʽ������������������Һ����������������
����⣺��1�������������ݿ�֪����һ�η�Ӧ��ȥ3.5g���壬�ڶ��Σ�ҲӦ��Ӧ��3.5g�������ϱ���n��ֵΪ13��
��2����ͼ�п�֪���ý�����ĩ��Al����������Ϊ
��100%=70%��
��3��������������Һ��������������Ϊx
2Al+2H2O+2NaOH=2NaAlO2+3H2��
54 80
20g-6g 80xg
=
���x=25.9%
������������Һ��������������Ϊ25.9%
�ʴ�Ϊ����1��13����2��70����3��25.9%
��2����ͼ�п�֪���ý�����ĩ��Al����������Ϊ
| 20g-6g |
| 20g |
��3��������������Һ��������������Ϊx
2Al+2H2O+2NaOH=2NaAlO2+3H2��
54 80
20g-6g 80xg
| 54 |
| 20g-6g |
| 80 |
| 80gx |
������������Һ��������������Ϊ25.9%
�ʴ�Ϊ����1��13����2��70����3��25.9%
������������Ҫ�����˽����Ļ�ѧ���ʺͽ������˳����Ӧ�ã������й��������������ļ�����ݻ�ѧ����ʽ�ļ��㣮

��ϰ��ϵ�д�
 �㽭֮��ѧҵˮƽ����ϵ�д�
�㽭֮��ѧҵˮƽ����ϵ�д� ��Ч���ܿ�ʱ��ҵϵ�д�
��Ч���ܿ�ʱ��ҵϵ�д�
�����Ŀ
��֪ij��Ͻ�����ĩ�г������������ͭ�е�һ�ֻ����֣�����������������5%���ϣ������ʵ��̽���÷�ĩ������ͭ�Ĵ��ڣ�
�������ߣ���������������Һ��Ӧ��������ˮ��ƫ�����ƺ�����
��2Al+2H2O+2NaOH=2NaAlO2+3H2����������ͭ��������������Һ��Ӧ��
�ɹ�ѡ���ʵ����Ʒ���ձ������Թܡ���Ͳ���ιܡ�ȼ�ճס�ҩ�ס�������ϡ���ᡢϡ���ᡢ
Na0H��Һ����ˮ��
������벢�������ʵ��̽�����̣�
��������� ����1���û�Ͻ�����ĩ�г��������Fe��
����2���û�Ͻ�����ĩ�г�������� ��
����3���û�Ͻ�����ĩ�г��������Fe��Cu��
����Ʋ�ʵʩʵ�鷽��
��1�����ڼ���1�����û�ѧ�Լ���֤���������Ĵ��ڿ��� ��
��2�����ڼ���2������Ͻ�����ĩ��������ϡ���ᣬ��۲쵽�������� ����Ӧ�Ļ�ѧ����ʽΪ ��
��3�����ڼ���3���������ʵ�����������ʵ������
����֪�ý�����ĩ�����Ϊ������������ͭ���������
��1��Ϊ�ⶨ��Ͻ�����ĩ������������������5.6g�Ľ�����ĩ�м���100gij��������������ϡ���ᣬǡ����ȫ��Ӧ��������a g������������˵����ȷ���� �� ����
A������ĩΪAl��Feʱ��a����Ϊ0.2g
B������ĩΪAl��Cuʱ����Ӧ��ȫ�����������Һ��һ������Al2��SO4��3��CuSO4
C������ĩΪAl��Cuʱ����aΪ0.3g������ַ�ĩ�к�������������С��50%
D������ĩΪAl��Feʱ������ϡ����������������һ������9.8%
��2����5.6g��ĩΪ����ͭ������97.6g������ϡ���ᣬ��ȫ��Ӧ��������0.3g��������Al2��SO4��3��Һ����������Al2��SO4��3��Է�������Ϊ342��Ҫ���м�����̣�
�������ߣ���������������Һ��Ӧ��������ˮ��ƫ�����ƺ�����
��2Al+2H2O+2NaOH=2NaAlO2+3H2����������ͭ��������������Һ��Ӧ��
�ɹ�ѡ���ʵ����Ʒ���ձ������Թܡ���Ͳ���ιܡ�ȼ�ճס�ҩ�ס�������ϡ���ᡢϡ���ᡢ
Na0H��Һ����ˮ��
������벢�������ʵ��̽�����̣�
��������� ����1���û�Ͻ�����ĩ�г��������Fe��
����2���û�Ͻ�����ĩ�г��������
����3���û�Ͻ�����ĩ�г��������Fe��Cu��
����Ʋ�ʵʩʵ�鷽��
��1�����ڼ���1�����û�ѧ�Լ���֤���������Ĵ��ڿ���
��2�����ڼ���2������Ͻ�����ĩ��������ϡ���ᣬ��۲쵽��������
��3�����ڼ���3���������ʵ�����������ʵ������
| ʵ�鲽�� | ʵ�������˵���������Լ��� | ʵ������ | ���� |
| �� | ȡ������ĩ������Թ��У����õιܵμ� |
�����ĩ ���� |
����ȥ�� |
| �� | �������Թ��м� |
���� ��Һ |
֤�������� |
| �� | �����Թܾ��ã���ȥ�ϲ���Һ����ˮ�����ϴ��ʣ����� | ʣ�������Ϻ�ɫ | ֤������ͭ |
��1��Ϊ�ⶨ��Ͻ�����ĩ������������������5.6g�Ľ�����ĩ�м���100gij��������������ϡ���ᣬǡ����ȫ��Ӧ��������a g������������˵����ȷ���� ��
A������ĩΪAl��Feʱ��a����Ϊ0.2g
B������ĩΪAl��Cuʱ����Ӧ��ȫ�����������Һ��һ������Al2��SO4��3��CuSO4
C������ĩΪAl��Cuʱ����aΪ0.3g������ַ�ĩ�к�������������С��50%
D������ĩΪAl��Feʱ������ϡ����������������һ������9.8%
��2����5.6g��ĩΪ����ͭ������97.6g������ϡ���ᣬ��ȫ��Ӧ��������0.3g��������Al2��SO4��3��Һ����������Al2��SO4��3��Է�������Ϊ342��Ҫ���м�����̣�
 ��2013?���ݣ��Ͻ���������Ӧ��ʮ�ֹ㷺����֪ij�Ͻ��ĩ�������⣬����������ͭ�е�һ�ֻ����֣�С��������ͼװ�öԺϽ��ĩ������ͭ�Ĵ��ڽ���̽��ʱ���������������Һ����ϡ������뵽��ƿ�У�������ķ�������ɫ����ų���ע�������������ƶ���
��2013?���ݣ��Ͻ���������Ӧ��ʮ�ֹ㷺����֪ij�Ͻ��ĩ�������⣬����������ͭ�е�һ�ֻ����֣�С��������ͼװ�öԺϽ��ĩ������ͭ�Ĵ��ڽ���̽��ʱ���������������Һ����ϡ������뵽��ƿ�У�������ķ�������ɫ����ų���ע�������������ƶ���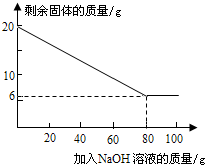 ����5%���ϣ������ʵ��̽���÷�ĩ������ͭ�Ĵ��ڣ�
����5%���ϣ������ʵ��̽���÷�ĩ������ͭ�Ĵ��ڣ� �Ͻ���������Ӧ��ʮ�ֹ㷺����֪ij�Ͻ��ĩ�����������⣬����������ͭ�е�һ�ֻ����֣�С��������ͼװ�öԺϽ��ĩ������ͭ�Ĵ��ڽ���̽��ʱ���������������Һ����ϡ������뵽��ƿ�У�������ķ�������ɫ����ų���ע�������������ƶ���
�Ͻ���������Ӧ��ʮ�ֹ㷺����֪ij�Ͻ��ĩ�����������⣬����������ͭ�е�һ�ֻ����֣�С��������ͼװ�öԺϽ��ĩ������ͭ�Ĵ��ڽ���̽��ʱ���������������Һ����ϡ������뵽��ƿ�У�������ķ�������ɫ����ų���ע�������������ƶ���