��Ŀ����
ijͬѧ����ͼ��ʾ��װ�ô��Եزⶨ���������������������ͼ���ձ��Ϸ�������(Ԥ�ȹ̶���)�в���һ���������һ����Ļ���������������ܷ��п����������Ҷ˵IJ����ܿڸ�������ͨ��ʵ�鿪ʼǰ�������ڿ̶�5 cm����(��֪�������Ż��Ϊ40������)

(1)ʵ�鷢��ʱ���ɹ۲쵽�������ڵ������ǣ��ٰ���(����)________���ڻ���________��
(2)ʵ����������º���Ӧͣ��Լ________cm�����ݴ˿ɵó��Ľ�����________��
�𰸣�
������
������
|
����(1)ȼ�ա������������������ƶ� ����(2)4������Լռ��������� |

��ϰ��ϵ�д�
�����Ŀ
 20��ij��ɫ������ֻ����̼��������Ԫ�أ�һ��ͬѧ����ͼ��ʾ��ʵ����̽��������ijɷ֣�ʵ���й۲쵽��ɫ������ɺ�ɫ�������ʯ��ˮ����ǣ�
20��ij��ɫ������ֻ����̼��������Ԫ�أ�һ��ͬѧ����ͼ��ʾ��ʵ����̽��������ijɷ֣�ʵ���й۲쵽��ɫ������ɺ�ɫ�������ʯ��ˮ����ǣ� ��2012?�ߴ���һģ��С��ͬѧ����ͼ��ʾװ�òⶨij������Ʒ������ΪNaCl����Na2CO3������������CΪ�п̶ȵIJ�����������װ���ʵ�Һ�壬����Һ��λ�ñ仯�ⶨ���������
��2012?�ߴ���һģ��С��ͬѧ����ͼ��ʾװ�òⶨij������Ʒ������ΪNaCl����Na2CO3������������CΪ�п̶ȵIJ�����������װ���ʵ�Һ�壬����Һ��λ�ñ仯�ⶨ���������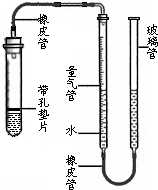 ij����С��ͬѧ����ͼ��ʾ��װ�ã��̶�װ��δ�������ⶨ�������ʵ�ʯ��ʯ��Ʒ��̼��Ƶ�������������������Ӵ����������壩��ʵ������ǣ�
ij����С��ͬѧ����ͼ��ʾ��װ�ã��̶�װ��δ�������ⶨ�������ʵ�ʯ��ʯ��Ʒ��̼��Ƶ�������������������Ӵ����������壩��ʵ������ǣ� ij����С��ͬѧ����ͼ��ʾ��װ�ã��̶�װ��δ�������ⶨ�������ʵ�ʯ��ʯ��Ʒ��̼��Ƶ�������������������Ӵ����������壩��ʵ������ǣ�
ij����С��ͬѧ����ͼ��ʾ��װ�ã��̶�װ��δ�������ⶨ�������ʵ�ʯ��ʯ��Ʒ��̼��Ƶ�������������������Ӵ����������壩��ʵ������ǣ�