��Ŀ����
ij����С��Ϊ�ⶨ����ʯ��ʯ�к�̼��Ƶ�����������ȡ����һЩ��ʯ����ȡϡ����200g��ƽ���ֳ�4�ݣ�����ʵ�飬������£�
��1����ʵ���У���
��2��m=
��3����ʯ��ʯ��CaCO3����������
| ʵ�� | 1 | 2 | 3 | 4 |
| ������Ʒ������/g | 5 | 10 | 15 | 20 |
| ����CO2������/g | 1.54 | 3.08 | 4.4 | m |
3��4
3��4
��ʵ����ʯ��ʯ��ʣ�࣬��1��2
1��2
��ʵ����������ʣ�࣮��2��m=
4.4
4.4
��3����ʯ��ʯ��CaCO3����������
70%
70%
����������1��ȡϡ����200 g��ƽ���ֳ�4�ݽ���ʵ�飬ÿ��ʵ��ʹ��ϡ����50g������ʵ�������ݱ���5gʯ��ʯ��ȫ��Ӧ�������ɶ�����̼1.54g����15gʯ��ʯ��ȫ��Ӧ���ɶ�����̼����=1.54g��3=4.62����ʵ���н��ų�4.4g������̼��˵�������μ�����Ʒʱʯ��ʯû����ȫ��Ӧ��û����ȫ��Ӧ������ϡ����㣻����3��ʵ���м�����Ʒ�����ɵĶ�����̼�������ӣ����Կ����ж�ǰ����ʵ����������ʣ�࣬���Ծݴ˽����⣻
��2�����ݣ�1���еķ�������֪������3�������������㣬���Ե�4�μ������Ʒ�����ٷ�Ӧ�����ɵ�������Ϊ4.4g�����Ծݴ˽��
��3������������������֪����һ�μ����ʯ��ʯ�е�̼�����ȫ��Ӧ�����Կ��Ը��ݵ�һ��ʵ�������ɵĶ�����̼��������Ϸ�Ӧ�Ļ�ѧ����ʽ���Լ�������ɵ�̼��Ƶ�������Ȼ����Լ����ʯ��ʯ������������
��2�����ݣ�1���еķ�������֪������3�������������㣬���Ե�4�μ������Ʒ�����ٷ�Ӧ�����ɵ�������Ϊ4.4g�����Ծݴ˽��
��3������������������֪����һ�μ����ʯ��ʯ�е�̼�����ȫ��Ӧ�����Կ��Ը��ݵ�һ��ʵ�������ɵĶ�����̼��������Ϸ�Ӧ�Ļ�ѧ����ʽ���Լ�������ɵ�̼��Ƶ�������Ȼ����Լ����ʯ��ʯ������������
����⣺��1��ȡϡ����200g��ƽ���ֳ�4�ݽ���ʵ�飬ÿ��ʵ��ʹ��ϡ����50g������ʵ�������ݱ���5gʯ��ʯ��ȫ��Ӧ�������ɶ�����̼1.54g����15gʯ��ʯ��ȫ��Ӧ���ɶ�����̼����=1.54g��3=4.62����ʵ���н��ų�4.4g������̼��˵�������μ�����Ʒʱʯ��ʯû����ȫ��Ӧ��û����ȫ��Ӧ������ϡ����㣻����3��ʵ���м�����Ʒ�����ɵĶ�����̼�������ӣ����Կ����ж�ǰ����ʵ����������ʣ�ࣻ
��2�����ݣ�1���еķ�������֪������3�������������㣬���Ե�4�μ������Ʒ�����ٷ�Ӧ�����ɵ�������Ϊ4.4g����m=4.4��
��3������ʯ��ʯ����̼��Ƶ���������Ϊx
CaCO3+2HCl�TCaCl2+H2O+CO2��
100 44
x 1.54g
=
��ã�x=3.5g
����ʯ��ʯ��̼��Ƶ���������Ϊ��
��100%=70%
��ʯ��ʯ��̼��Ƶ���������Ϊ70%
�ʴ�Ϊ����1��3��4��1��2��
��2��4.4��
��3��70%��
��2�����ݣ�1���еķ�������֪������3�������������㣬���Ե�4�μ������Ʒ�����ٷ�Ӧ�����ɵ�������Ϊ4.4g����m=4.4��
��3������ʯ��ʯ����̼��Ƶ���������Ϊx
CaCO3+2HCl�TCaCl2+H2O+CO2��
100 44
x 1.54g
| 100 |
| x |
| 44 |
| 1.54g |
��ã�x=3.5g
����ʯ��ʯ��̼��Ƶ���������Ϊ��
| 3.5g |
| 5g |
��ʯ��ʯ��̼��Ƶ���������Ϊ70%
�ʴ�Ϊ����1��3��4��1��2��
��2��4.4��
��3��70%��
���������⿼����ݻ�ѧ����ʽ���м��㣬Ҫ�����������Ŀ�����ȣ�Ҫ�������Ǹ��ݻ�ѧ��Ӧ����ʽ�ļ��㲽���ʽ���Լ���֮��ص�֪ʶ�ȣ����������ѡ����𣬱���ؼ����жϷ�Ӧ�Ƿ���ȫ��

��ϰ��ϵ�д�
 ��ʦ������Ԫ��ĩ���100��ϵ�д�
��ʦ������Ԫ��ĩ���100��ϵ�д� ��У������Ԫͬ��ѵ��������ϵ�д�
��У������Ԫͬ��ѵ��������ϵ�д�
�����Ŀ
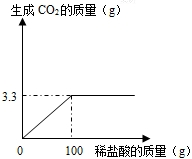 ij����С��Ϊ�ⶨ����ʯ��ʯ�к�̼��Ƶ�����������ȡ����һЩ��ʯ��ȷ��ȡ��Ʒ10g�����ձ��У����ʼȲ�����ˮ��Ҳ����ϡ���ᷴӦ��Ҳ���ֽ⣩�������м���������ϡ���ᣬ����ʵ���õ����ݻ�����ͼ��
ij����С��Ϊ�ⶨ����ʯ��ʯ�к�̼��Ƶ�����������ȡ����һЩ��ʯ��ȷ��ȡ��Ʒ10g�����ձ��У����ʼȲ�����ˮ��Ҳ����ϡ���ᷴӦ��Ҳ���ֽ⣩�������м���������ϡ���ᣬ����ʵ���õ����ݻ�����ͼ��