��Ŀ����
��2012?����ģ�⣩��1�����Ƽ���H1N1������ЧҩƷ����ơ�����ʼԭ����ç���ᣨC7H10O5����ç������
��2����6�֣�ij�������IJ�Ʒ̼�����л�������̼�����ƣ�Ϊ�˲ⶨ��Ʒ��̼���Ƶ�����������ȡ100g��Ʒ���ȣ�2NaHCO3
Na2CO3+H2O+CO2����̼�������Ȳ��ֽ⣩����ȫ�ֽ����ɶ�����̼����0.22g������Ʒ��̼���Ƶ�����������
3
3
��Ԫ����ɣ������⡢��Ԫ�ص�������Ϊ1��8
1��8
����2����6�֣�ij�������IJ�Ʒ̼�����л�������̼�����ƣ�Ϊ�˲ⶨ��Ʒ��̼���Ƶ�����������ȡ100g��Ʒ���ȣ�2NaHCO3
| ||
��������1����ç����Ļ�ѧʽC7H10O5��֪��ç������C��H��OԪ����ɣ�ÿ��ç��������к�7��Cԭ�ӡ�10��Hԭ�ӡ�5��Oԭ�ӣ��ݴ˿ɼ���ç������H��OԪ�������Ƚ��н��
��2������̼���������ȷֽ�Ļ�ѧ����ʽ���ɲ���������̼����������ֽ��̼�����Ƶ���������Ʒ������̼�����������IJ��Ʒ��̼���Ƶ�����������������Ʒ�����ȿɼ���100g�������̼���Ƶ������������н��
��2������̼���������ȷֽ�Ļ�ѧ����ʽ���ɲ���������̼����������ֽ��̼�����Ƶ���������Ʒ������̼�����������IJ��Ʒ��̼���Ƶ�����������������Ʒ�����ȿɼ���100g�������̼���Ƶ������������н��
����⣺��1����ç����Ļ�ѧʽC7H10O5��֪��ç������C��H��O����Ԫ����ɣ�����H��OԪ��������=��1��10������16��5��=1��8��
�ʴ�Ϊ��3��1��8��
��2������Ʒ��̼�����Ƶ�����Ϊx
2NaHCO3
Na2CO3+H2O+CO2��
168 44
x 0.22g
=
x=0.84g
��Ʒ��̼���Ƶ���������=
��100%=99.16%
����Ʒ��̼���Ƶ���������Ϊ99.16%��
�ʴ�Ϊ��3��1��8��
��2������Ʒ��̼�����Ƶ�����Ϊx
2NaHCO3
| ||
168 44
x 0.22g
| 168 |
| x |
| 44 |
| 0.22g |
x=0.84g
��Ʒ��̼���Ƶ���������=
| 100g-0.84g |
| 100g |
����Ʒ��̼���Ƶ���������Ϊ99.16%��
�����������ǶԻ�ѧ����ʽ����Ŀ��飬����Ĺؼ����ҵ���֪���������������÷�Ӧ���ɶ�����̼����������������ʵ�������

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
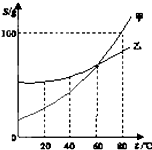 ��2012?����ģ�⣩��ͼΪ�ס����������ʵ��ܽ�����ߣ����ͼʾ�ش��������⣺
��2012?����ģ�⣩��ͼΪ�ס����������ʵ��ܽ�����ߣ����ͼʾ�ش��������⣺