��Ŀ����
�������岻��ȱ�ٵ���Ԫ�أ�ȱ��������ƶѪ��������ÿ��������ȡ14mg���ҵ������ڽ����м�������ǿ������������[��ѧʽΪ��C3H5O3��2Fe]�����ҹ�Ϊ���ȱ����ƶѪ��ʵʩ����Ŀ����C-12 H-1��O=16��Fe-56��
��1��������������Է�������Ϊ________��
��2��������������Ԫ�ص���������Ϊ________��
��3�����ҹ涨��ǿ�������������ĺ���Ϊ22.4mg/100mL���ң���ÿ1L��ǿ����������Ӧ����������������ԼΪ________g��
�⣺��1��������������Է�������Ϊ12��3��2+1��5��2+16��3��2+56=234��
��2��������������Ԫ�ص���������Ϊ 100%=23.9%��
100%=23.9%��
��3���������⣬���ҹ涨��ǿ�������������ĺ���Ϊ22.4mg/100mL���ң���1L��ǿ���������к���������Ϊ224mg��224mg=0.224g����������������������Ϊ0.224g��23.9%=0.936g��
�ʴ�Ϊ����1��234����2��23.9%����3��0.936��
��������1��������Է�������Ϊ��ɷ��ӵĸ�ԭ�ӵ����ԭ������֮�ͣ����з������
��2�����ݻ�������Ԫ�ص���������=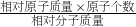 ��100%�����з������
��100%����������
��3���������⣬���ҹ涨��ǿ�������������ĺ���Ϊ22.4mg/100mL���ң����Լ����1L��ǿ���������к������������������ݻ�������ijԪ�ص�����=�û��������������Ԫ�ص���������������������������������ɣ�
�����������ѶȲ�����ͬѧ�ǽ������Ϣ��������û�ѧʽ���йؼ�����з������⡢��������������
��2��������������Ԫ�ص���������Ϊ
 100%=23.9%��
100%=23.9%����3���������⣬���ҹ涨��ǿ�������������ĺ���Ϊ22.4mg/100mL���ң���1L��ǿ���������к���������Ϊ224mg��224mg=0.224g����������������������Ϊ0.224g��23.9%=0.936g��
�ʴ�Ϊ����1��234����2��23.9%����3��0.936��
��������1��������Է�������Ϊ��ɷ��ӵĸ�ԭ�ӵ����ԭ������֮�ͣ����з������
��2�����ݻ�������Ԫ�ص���������=
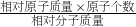 ��100%����������
��100%�����з��������3���������⣬���ҹ涨��ǿ�������������ĺ���Ϊ22.4mg/100mL���ң����Լ����1L��ǿ���������к������������������ݻ�������ijԪ�ص�����=�û��������������Ԫ�ص���������������������������������ɣ�
�����������ѶȲ�����ͬѧ�ǽ������Ϣ��������û�ѧʽ���йؼ�����з������⡢��������������

��ϰ��ϵ�д�
 ȫ�ŵ�����Ԫ�ƻ�ϵ�д�
ȫ�ŵ�����Ԫ�ƻ�ϵ�д�
�����Ŀ


 2H2��+O2��
2H2��+O2��