��Ŀ����
ˮ�DZ������Ȼ��Դ���ڹ�ũҵ�������ճ����������ż���㷺�����ã���ͼΪ����ˮ����ˮ����ʾ��ͼ��
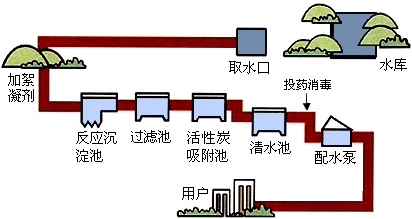
��1��������ˮ����������ˮʱ��ʹ�õľ�ˮ������
A������ B������ C����� D������ E������
��2��ȡˮ�������������������������
��3����ͼ�������ڵĻ���̿���������ã������������˵Ⱦ������������õ�ˮ
��4����ͥ�����п�����
��5����Щ��ѧ��Ԥ�ԣ������������һ��ˮ������������ᡱ����仰��ʾ����Ӧ��������ˮ��Դ����ʶ��һ�ǽ�Լ��ˮ�����Ƿ�ֹˮ����Ⱦ�������һ����Լ��ˮ��������

��1��������ˮ����������ˮʱ��ʹ�õľ�ˮ������
A��B��E
A��B��E
��A������ B������ C����� D������ E������
��2��ȡˮ�������������������������
ʹ������ˮ�е��������۳���
ʹ������ˮ�е��������۳���
����3����ͼ�������ڵĻ���̿���������ã������������˵Ⱦ������������õ�ˮ
����
����
��ˮ����ǡ����ǡ�������4����ͥ�����п�����
����ˮ
����ˮ
����ijˮ����Ӳˮ������ˮ����5����Щ��ѧ��Ԥ�ԣ������������һ��ˮ������������ᡱ����仰��ʾ����Ӧ��������ˮ��Դ����ʶ��һ�ǽ�Լ��ˮ�����Ƿ�ֹˮ����Ⱦ�������һ����Լ��ˮ��������
ũҵ����Ľ���Ϊ���
ũҵ����Ľ���Ϊ���
�������𰸾��ɣ�����������1����������ˮ�ľ������̽��н��
��2���������������ý��н��
��3����������̿������ˮ���������е����ã����жϾ����������˺��ˮ�Dz��Ǵ����
��4��ѡ��������Ʒ����Ӳˮ����ˮ��
��5������˵����Լ��ˮ�ķ�����
��2���������������ý��н��
��3����������̿������ˮ���������е����ã����жϾ����������˺��ˮ�Dz��Ǵ����
��4��ѡ��������Ʒ����Ӳˮ����ˮ��
��5������˵����Լ��ˮ�ķ�����
����⣺��1��������ˮ����������ˮʱ��ʹ�õľ�ˮ�����г��������ˡ�������
��2��ȡˮ������������������������� ʹ������ˮ�е��������۳�����
��2������̿���ж�ṹ���������ж��к����ʣ�������ˮ������������ˮ���ж��к���ζ���ʵ����ã������������˵�ˮֻ�dz�ȥ��ˮ�в����Թ������ʣ��Ժ���������������ʣ����Եõ��IJ��Ǵ����
��4����ˮ�е������ˮ������ḻ����ĭ����Ӳˮ�������ˮ��������ĭ�������и��������Գ��÷���ˮ������ijˮ����Ӳˮ������ˮ��
��5����Լ��ˮӦ��ÿ�������𣬿ɽ�������еľ���������˵����ν�Լ��ˮ����ũҵ����Ľ���Ϊ���ȣ�
�ʴ�Ϊ����1��ABE����2��ʹ������ˮ�е��������۳�������3�����ǣ���4������ˮ����5��ũҵ����Ľ���Ϊ��࣮
��2��ȡˮ������������������������� ʹ������ˮ�е��������۳�����
��2������̿���ж�ṹ���������ж��к����ʣ�������ˮ������������ˮ���ж��к���ζ���ʵ����ã������������˵�ˮֻ�dz�ȥ��ˮ�в����Թ������ʣ��Ժ���������������ʣ����Եõ��IJ��Ǵ����
��4����ˮ�е������ˮ������ḻ����ĭ����Ӳˮ�������ˮ��������ĭ�������и��������Գ��÷���ˮ������ijˮ����Ӳˮ������ˮ��
��5����Լ��ˮӦ��ÿ�������𣬿ɽ�������еľ���������˵����ν�Լ��ˮ����ũҵ����Ľ���Ϊ���ȣ�
�ʴ�Ϊ����1��ABE����2��ʹ������ˮ�е��������۳�������3�����ǣ���4������ˮ����5��ũҵ����Ľ���Ϊ��࣮
���������⿼������ݲ��ѣ����漰�����ݽ϶࣬����һ����İ���ȣ�������ܺܺõ���չӦ�䣬�����Ļ���ͺܴ����Ҫϸ�µķ�����

��ϰ��ϵ�д�
�����Ŀ

 ��2012?��ɽ��һģ��ˮ�DZ������Ȼ��Դ���ڹ�ũҵ�������ճ��������й㷺��Ӧ�ã�
��2012?��ɽ��һģ��ˮ�DZ������Ȼ��Դ���ڹ�ũҵ�������ճ��������й㷺��Ӧ�ã�