��Ŀ����
������ͼװ��

��1��д��ͼ�б������������ ��________ ��________ ��________
��2��ʵ������ȡ������̼����Ļ�ѧ����ʽΪ________����ȷ�ķ���װ�ú��ռ�װ���ǣ�����ţ�________��
��3���ڳ��л�ѧ�����Aװ�ý��е�ʵ���У�ֻҪд��������ͬ�Ļ�ѧ����ʽ��________��________��
��4��С��ͬѧ��ʵ�������ù�������������������д���÷�Ӧ�Ļ�ѧ����ʽ
________����ѡ����Cװ�ã�����Ϊ________����С����С����������˵�����ɣ�������ͼ��ԸĽ�________��
�⣺��1����������̨���������̨��
���dz���©�����������©����
����ˮ�ۣ����ˮ�ۣ�
��2��ʯ��ʯ��ϡ���ᷴӦ�Ļ�ѧ����ʽΪ��CaCO3+2HCl=CaCl2+H2O+CO2����
ʯ��ʯ��ϡ���ᷴӦ����Ҫ���ȣ����ɵĶ�����̼���ܶȱȿ��������������ſ������ռ������BD��
��3������������ȷֽ�Ļ�ѧ����ʽΪ��2KMnO4 K2MnO4+MnO2+O2����
K2MnO4+MnO2+O2����
̼��������ȷֽ�Ļ�ѧ����ʽΪ��NH4HCO3 NH3��+H2O+CO2����
NH3��+H2O+CO2����
��4�����������ڶ������̵Ĵ������£��ֽ�������ˮ����������Ӧ�Ļ�ѧ����ʽΪ��2H2O2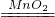 2H2O+O2����
2H2O+O2����
Cװ���еij���©��ĩ����Һ�����ϣ����ɵ�������ӳ���©���ݳ����ռ�����������������У�Ӧ�ý�����©���¶��쵽Һ�����£�
��������1��Ҫ���ո������������ƺ���;��
��2��ʵ���ҿ�����ʯ��ʯ��ϡ���ᷴӦ��ȡ������̼��
��3�����ȸ��������ȡ����������̼����淋ȿ�����Aװ�ý��У�
��4����������ֽ�������ˮ��������Ҫ��ȷ������ʵ��װ�ã�
������������Ҫ������ȡ���塢�ռ�����ķ����ͻ�ѧ����ʽ����д�ȷ����֪ʶ����д��ѧ����ʽʱҪע����ѭ�����غ㶨�ɣ�
���dz���©�����������©����
����ˮ�ۣ����ˮ�ۣ�
��2��ʯ��ʯ��ϡ���ᷴӦ�Ļ�ѧ����ʽΪ��CaCO3+2HCl=CaCl2+H2O+CO2����
ʯ��ʯ��ϡ���ᷴӦ����Ҫ���ȣ����ɵĶ�����̼���ܶȱȿ��������������ſ������ռ������BD��
��3������������ȷֽ�Ļ�ѧ����ʽΪ��2KMnO4
 K2MnO4+MnO2+O2����
K2MnO4+MnO2+O2����̼��������ȷֽ�Ļ�ѧ����ʽΪ��NH4HCO3
 NH3��+H2O+CO2����
NH3��+H2O+CO2������4�����������ڶ������̵Ĵ������£��ֽ�������ˮ����������Ӧ�Ļ�ѧ����ʽΪ��2H2O2
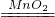 2H2O+O2����
2H2O+O2����Cװ���еij���©��ĩ����Һ�����ϣ����ɵ�������ӳ���©���ݳ����ռ�����������������У�Ӧ�ý�����©���¶��쵽Һ�����£�
��������1��Ҫ���ո������������ƺ���;��
��2��ʵ���ҿ�����ʯ��ʯ��ϡ���ᷴӦ��ȡ������̼��
��3�����ȸ��������ȡ����������̼����淋ȿ�����Aװ�ý��У�
��4����������ֽ�������ˮ��������Ҫ��ȷ������ʵ��װ�ã�
������������Ҫ������ȡ���塢�ռ�����ķ����ͻ�ѧ����ʽ����д�ȷ����֪ʶ����д��ѧ����ʽʱҪע����ѭ�����غ㶨�ɣ�

��ϰ��ϵ�д�
 ���ѵ����Ԫ��ĩ���100��ϵ�д�
���ѵ����Ԫ��ĩ���100��ϵ�д� ��˼άС�ھ�100����ҵ��ϵ�д�
��˼άС�ھ�100����ҵ��ϵ�д� ��ʦָ��һ��ͨϵ�д�
��ʦָ��һ��ͨϵ�д�
�����Ŀ

 ��ͼ��ʵ�����ø��������������װ��ͼ���������ͼ���
��ͼ��ʵ�����ø��������������װ��ͼ���������ͼ���
