��Ŀ����
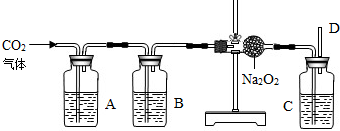
�������ƣ�Na2O2����һ�ֻ�ѧ���ʻ��õĵ���ɫ���壬��ͨ��״�����ܸ��������ʷ�����ѧ��Ӧ�ų����������磺2Na2O2+2CO2�T2Na2CO3+O2����2Na2O2+4HCl=4NaCl+2H2O+O2����2Na2O2+2H2O=4NaOH+O2���ȣ�С��Ϊ����֤CO2��Na2O2��Ӧʱ���������������������������ʵ��װ�ã�
�Իش�
��1����ʯ��ʯ��������ȡʵ�����õ�CO2�����A��Bװ�õ�Ŀ���Ǿ��������CO2������Aƿ�ڵ�Һ������������Һ����������______���������Ļ�ѧ��Ӧ����ʽΪ______��Bװ����Һ��Ӧ��______��
��2��Cƿ��Һ��Ӧ��______��
��3����Ӧ��ϣ����ʢNa2O2�ķ�Ӧ����������������14g�������������������Ϊ______g��

�Իش�
��1����ʯ��ʯ��������ȡʵ�����õ�CO2�����A��Bװ�õ�Ŀ���Ǿ��������CO2������Aƿ�ڵ�Һ������������Һ����������______���������Ļ�ѧ��Ӧ����ʽΪ______��Bװ����Һ��Ӧ��______��
��2��Cƿ��Һ��Ӧ��______��
��3����Ӧ��ϣ����ʢNa2O2�ķ�Ӧ����������������14g�������������������Ϊ______g��
��1��Aƿ�ڵ���������Һ�������Ȼ������巢�����ֽⷴӦ����ȥ���ڶ�����̼�����е��Ȼ������壮�ʴ𣺳�ȥ������̼�е��Ȼ������壻HCl+AgNO3=AgCl��+HNO3��
��ȥ������̼�����е�ˮ��ͨ��ѡ��Ũ���ᣮ�ʴ�Ũ���
��2��Cƿ�����ó�ȥ���ɵ������л��еĶ�����̼�����ö�����̼�����Һ��Ӧ��ͨ��ѡ��������մ���������̼���������������Һ���ʴ�����������Һ��
��3����ʢNa2O2�ķ�Ӧ����������������14g�����������ӵ�ԭ�������й����ĩ���ն�����̼������ų�����������������������������Ϊx�������ն�����̼������Ϊ14g+x�����ݻ�ѧ����ʽ���м�����xֵ��
2Na2O2+2CO2�T2Na2CO3+O2��
88 32
14g+x x
=
x=8g
�ʴ�8g��
��ȥ������̼�����е�ˮ��ͨ��ѡ��Ũ���ᣮ�ʴ�Ũ���
��2��Cƿ�����ó�ȥ���ɵ������л��еĶ�����̼�����ö�����̼�����Һ��Ӧ��ͨ��ѡ��������մ���������̼���������������Һ���ʴ�����������Һ��
��3����ʢNa2O2�ķ�Ӧ����������������14g�����������ӵ�ԭ�������й����ĩ���ն�����̼������ų�����������������������������Ϊx�������ն�����̼������Ϊ14g+x�����ݻ�ѧ����ʽ���м�����xֵ��
2Na2O2+2CO2�T2Na2CO3+O2��
88 32
14g+x x
| 88 |
| 14+x |
| 32 |
| x |
�ʴ�8g��

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ



 23��������ߺ�DZˮͧ�п��ù������ƣ�Na2O2����Ϊ����������������ȤС��ͬѧΧ�ƹ������ƽ��е�һϵ��̽����������뵽���У�
23��������ߺ�DZˮͧ�п��ù������ƣ�Na2O2����Ϊ����������������ȤС��ͬѧΧ�ƹ������ƽ��е�һϵ��̽����������뵽���У�
