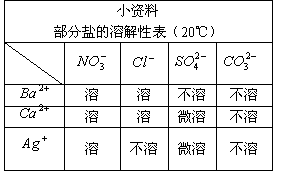
��Ŀ����
С��ϲ�������㣬�������������ϵ�֪�������ÿ��ˮ������������0.003gʱ����ͻ��������������ƣ�CaO2����һ�ֻ�ѧ���������䷴Ӧԭ����2CaO2+2H2O====2Ca(OH) 2+O2����ش�
��1��������ˮ�е��ܽ�����¶ȵ����߶� ����������
��2��С����2.88g�������Ʒ���ʢ��200Lˮ������У�����ͨ�������������������ȫ�ͷź������ˮ���������Ƿ�������Ҫ��(���������������ȫ����ˮ���������غ��Բ���)
��1����С 1��
��2���⣺��2.88gCaO2��ȫ��Ӧ����O2������Ϊx.
2CaO2 + 2H2O = Ca(OH)2 + O2��
144 32
2.88 g x

 =
= 1��
1��
 x=0.64g 1��
x=0.64g 1��
�������ˮ��������Ϊ0.64g��200L=0.0032g/L 1��
��Ϊ0.0032g/L>0.003g/L
���������ˮ���������������Ҫ�� 1��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д���Ӣͬѧȡij��ʯ��ʯ��Ʒ12g���вⶨʵ�飬�ֽ�100gϡ�������μ���ʯ��ʯ��Ʒ�У����ʲ�����ˮҲ�����뷴Ӧ������ַ�Ӧ����������������������±���ʾ��
| ��1�� | ��2�� | ��3�� | ��4�� | ��5�� | |
| ����ϡ���������/g | 20 | 20 | 20 | 20 | 20 |
| ���������������/g | 1.1 | 2.2 | m | 4.4 | 4.4 |
����
��1��m��ֵΪ g��1�֣�
��2��12gʯ��ʯ��Ʒ��̼��Ƶ��������� g��2�֣�
��3����Ӧ��ȫ��������Һ���Ȼ��Ƶ����������� ��3�֣�
��д��������̣���������ȷ��0.1��
 ��Һ�����ó������������NaOH��Һ��������ϵ��ͼ��ʾ
��Һ�����ó������������NaOH��Һ��������ϵ��ͼ��ʾ ��1������������������Ϊ14.6%��ϡ���ᣬ��Ҫ��������Ϊ36.5%��Ũ�����������______��
��1������������������Ϊ14.6%��ϡ���ᣬ��Ҫ��������Ϊ36.5%��Ũ�����������______��