��Ŀ����
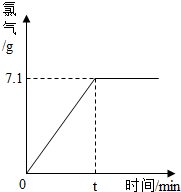
��ͼ2Ϊ�Ȼ��ƺ�����ع�����ܽ�����ߣ�����۲��ͼ���ش������й����⣺
��1�����ݸ�ͼ��֪���Ȼ��Ƶ��ܽ�����¶�Ӱ��仯�����ɸ�ͼ�㻹�����Щ��Ϣ����д��������
��2������t��ʱ����ر�����Һת��Ϊ��������Һ�������
��3��ij��Ϊ�˼����ˮ���ж��������ӵĺ�����������l00g 1l%���Ȼ�����Һ��ijѧ������ͼ1��ʾ�������������Ϊ���ϲ������д�����ǣ�����ţ�

��1�����ݸ�ͼ��֪���Ȼ��Ƶ��ܽ�����¶�Ӱ��仯�����ɸ�ͼ�㻹�����Щ��Ϣ����д��������
����ص��ܽ�����¶ȵ�Ӱ��ϴ�
����ص��ܽ�����¶ȵ�Ӱ��ϴ�
����t��ʱ���Ȼ��ƺ�����ص��ܽ����ȣ�
��t��ʱ���Ȼ��ƺ�����ص��ܽ����ȣ�
��2������t��ʱ����ر�����Һת��Ϊ��������Һ�������
��ˮ
��ˮ
������
����
���ַ�������3��ij��Ϊ�˼����ˮ���ж��������ӵĺ�����������l00g 1l%���Ȼ�����Һ��ijѧ������ͼ1��ʾ�������������Ϊ���ϲ������д�����ǣ�����ţ�
A��B
A��B
�������������һ�������������NaCl�����̣���������̣���ȡ11gNaCl
��NaCl�����̣���������̣���ȡ11gNaCl
����������1����2�����ܽ�����ߣ�
���ж�����ػ��Ȼ��������ڲ�ͬ�¶��µ��ܽ�ȴ�С��
�ڱȽϲ�ͬ������ͬһ�¶��µ��ܽ�ȴ�С��
���ж����ʵ��ܽ�����¶�Ӱ��仯�����ƣ�
�����ͨ���ı��¶Ȼ��������ʽ���������Һ��Ϊ������Һ��
��3������100g 11%���Ȼ�����Һ����Ҫ��������-����-�ܽ�Ĺ��̣����ݹ����еIJ���Ҫ���ж�ͼʾ�����еIJ��������Ը�����
���ж�����ػ��Ȼ��������ڲ�ͬ�¶��µ��ܽ�ȴ�С��
�ڱȽϲ�ͬ������ͬһ�¶��µ��ܽ�ȴ�С��
���ж����ʵ��ܽ�����¶�Ӱ��仯�����ƣ�
�����ͨ���ı��¶Ȼ��������ʽ���������Һ��Ϊ������Һ��
��3������100g 11%���Ȼ�����Һ����Ҫ��������-����-�ܽ�Ĺ��̣����ݹ����еIJ���Ҫ���ж�ͼʾ�����еIJ��������Ը�����
����⣺��1�������ܽ������ͼ�ı�ʾ���壬���ǿ����жϳ�������ص��ܽ�����¶ȵ�Ӱ��ϴ���t��ʱ�����ߵ��ܽ����ȣ��ʴ�Ϊ������ص��ܽ�����¶ȵ�Ӱ��ϴ���t��ʱ���Ȼ��ƺ�����ص��ܽ����ȣ�
��2����������ص��ܽ�����¶�Ӱ��ϴʴ�Ϊ����ˮ�����£�
��3��ʹ��������ƽ���г���ʱ��Ӧ��ѭ���������롱��ʹ��ԭ��ͼʾ�ij�ȡ�Ȼ��ƵIJ������Ȼ���������Ŵ���λ�ã�ʹ����Ͳ��ȡˮ��������Ӧ�밼Һ����ʹ�����ˮƽ��ͼʾ��ȡˮ�IJ����д���ز�ȡ�����Ӷ������ʴ�Ϊ��A��B����NaCl�����̣���������̣���ȡ11gNaCl��
��2����������ص��ܽ�����¶�Ӱ��ϴʴ�Ϊ����ˮ�����£�
��3��ʹ��������ƽ���г���ʱ��Ӧ��ѭ���������롱��ʹ��ԭ��ͼʾ�ij�ȡ�Ȼ��ƵIJ������Ȼ���������Ŵ���λ�ã�ʹ����Ͳ��ȡˮ��������Ӧ�밼Һ����ʹ�����ˮƽ��ͼʾ��ȡˮ�IJ����д���ز�ȡ�����Ӷ������ʴ�Ϊ��A��B����NaCl�����̣���������̣���ȡ11gNaCl��
���������⿼���˴��ܽ������ͼ�϶�ȡ��Ϣ����������ȡ��Ϣ����������ȡˮʱ���Ӷ���������С������ȡˮ��ʵ�������ʹ��ȡˮ�����ƫ���������Ȼ�����Һ����������ƫС��

��ϰ��ϵ�д�
�����Ŀ
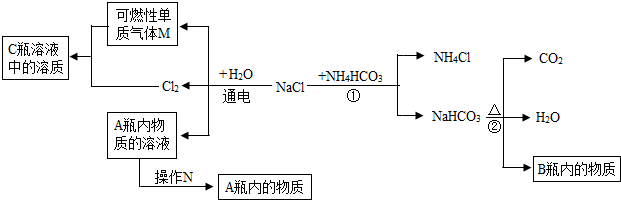
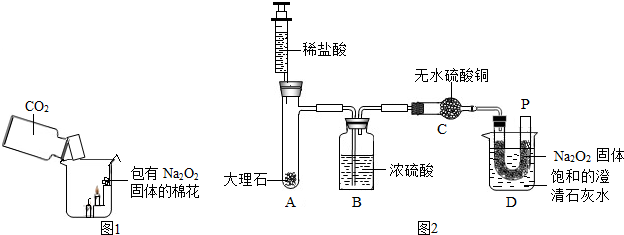
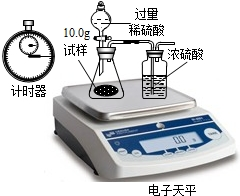
 �ڻ��������У�ԭ����������ȫ��ת���ɲ�Ʒ��ijУѧ���������Ĺ�ҵ�����ռ�����ʳɷֽ����о������Dz������ϵ�֪���ڹ�ҵ����ʳ�κ�ˮ��ɱ���ʳ��ˮ��ͨ����⣨ͨ�磩�Ƶ��ռ���Һ���ٽ���ҺŨ�����ɵõ������ռͨ������л��ɵõ�������������Cl2���������Ʋ�ù�ҵ�����ռ��п��ܺ���̼���ơ��Ȼ��ƣ��������ʵ������֤���裮
�ڻ��������У�ԭ����������ȫ��ת���ɲ�Ʒ��ijУѧ���������Ĺ�ҵ�����ռ�����ʳɷֽ����о������Dz������ϵ�֪���ڹ�ҵ����ʳ�κ�ˮ��ɱ���ʳ��ˮ��ͨ����⣨ͨ�磩�Ƶ��ռ���Һ���ٽ���ҺŨ�����ɵõ������ռͨ������л��ɵõ�������������Cl2���������Ʋ�ù�ҵ�����ռ��п��ܺ���̼���ơ��Ȼ��ƣ��������ʵ������֤���裮��1����������������ʵ�鱨�棺
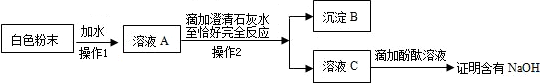
| ʵ�鲽�� | ʵ������ | �� �� |
| ��ȡ������������ˮ | ������ȫ�ܽ� | -- |
| �����Թ�ȡ������Һ���������ϡ���ᣬ���ϴ����ܵĵ������������ܵ���һ�˲��� |
�� �� |
֤��ԭ�����ռ��к���̼���� |
| �����Թ����ټ��������� | �а�ɫ�������� | ֤��ԭ�����ռ��к��� |
�� 3 ����ȡ100g�����µı���ʳ��ˮ����ʱ��������ԼΪ26.5%�����е�⣬����Ӧֹͣ������������������ʱ��Ĺ�ϵ��ͼ��ʾ������㣺�μӷ�Ӧ���Ȼ��Ƶ������Ƕ��٣���Ӧ�������ռ���Һ�����������Ƕ��٣�����������ȷ��0.001��




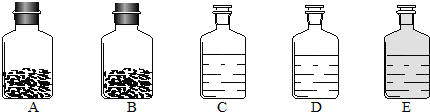
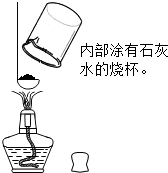 ��ʵ��һ����װ�����۲�������ʣ�����������ҪΪ��ɫ��ĩ�����л��С�����ɫ������С����
��ʵ��һ����װ�����۲�������ʣ�����������ҪΪ��ɫ��ĩ�����л��С�����ɫ������С����