��Ŀ����
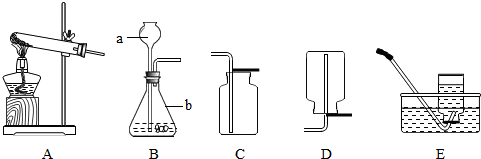
��8�֣�ij��ѧ��ȤС��������ͼװ��̽���������ȡԭ�������������ʡ����װ��ͼ���ش��������⣺

(1)д����ͼ������a�����ƣ� ��ʵ������ȡ������Ӧѡ���װ���� (����ͼ��ĸ)��
(2) ��װ��A��ȡ�����Ļ�ѧ����ʽΪ ����Eװ���ռ�����������ԭ������� ��дһ������
(3)С����װ��F̽��CO2��ijЩ���ʣ�����������CO2ͨ��װ��F�۲쵽:�������沣 ������ʪ���ʯ����ֽ��죬���沣���������������ݴ�˵��������̼�������У��� ���� ��
��1����ƿ��BE
��2��2KMnO4 K2MnO4
+MnO2+ O2����2KClO3
K2MnO4
+MnO2+ O2����2KClO3 2KCl+3
O2
2KCl+3
O2 ��
��
�ռ�ǰ����ƿû��ʢ��ˮ�ܿ�һ������ð���Ϳ�ʼ�ռ�
��3��������̼���ܶȱȿ���������̼�ܺ�ˮ��Ӧ������������
����������1������װ��ͼ�����������ã���ʶ������������ȷд�����������ƣ�
�����ķ�Ӧ�ڳ����¾��ܷ�������˲���Ҫ����
��2���ø��������ȡ�����Ǽ��ȹ��������壻��ˮ���ռ�����ʱ�����ռ�ǰ����ƿ�е�ˮû��װ�����������ݣ����ռ����������п�������û��װ���еĿ����ž��������ռ�����û�ȵ��ܿ��������Ҿ��ȵ�����ð���Ϳ�ʼ�ռ���������������Ҳ���п�����
��3���ɹ۲쵽���������沣������ʪ���ʯ����ֽ��죬���沣���������������������˵����������̼�����ܶȱȿ����ܶȴ�����̼��ˮ��Ӧ�����������ԣ�

 Сѧ��10���ӿ������100��ϵ�д�
Сѧ��10���ӿ������100��ϵ�д�


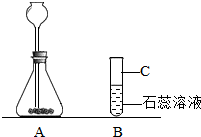


 ��2007?���ij��ѧ��ȤС��������ͼװ�ý��б���NaCl��Һ�ĵ��ʵ�飬���Բ��ֲ������̽����
��2007?���ij��ѧ��ȤС��������ͼװ�ý��б���NaCl��Һ�ĵ��ʵ�飬���Բ��ֲ������̽����