��Ŀ����
��ѡ�����������ش����⣺
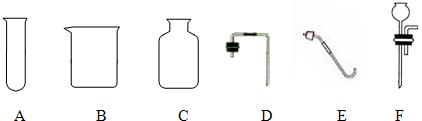
��1����������A����һ���ø��������ȡ���ռ�������װ�ã�����ѡ�������������е�
������ĸ��������װ���л�ȱ�ٵ������� ��
��2��ʵ������������A��ȡ���ռ�������̼������Ҫѡ�õ������� ������ĸ������Ӧ�Ļ�ѧ����ʽ�� ������������Ƿ��ռ����ķ����� ��
��3��������A��һ���ײ���С���Թܣ�������װ�ɿ�����ʱ��Ӧ����ʱֹͣ����ȡ������̼�ķ���װ�ã�����Ҫѡ�����������е� ��
��1��C E �ƾ���
��2��C D����CF�� CaCO3 + 2HCl == CaCl2 + H2O + CO2�� ��ȼ�ŵ�ľ������ƿ�ڣ���Ϩ��������
��3��B D �ձ�

��ϰ��ϵ�д�
�����Ŀ
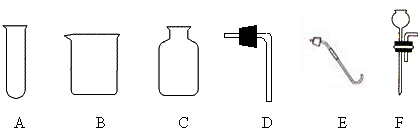
 ��ѡ�����������ش����⣺
��ѡ�����������ش����⣺